 |
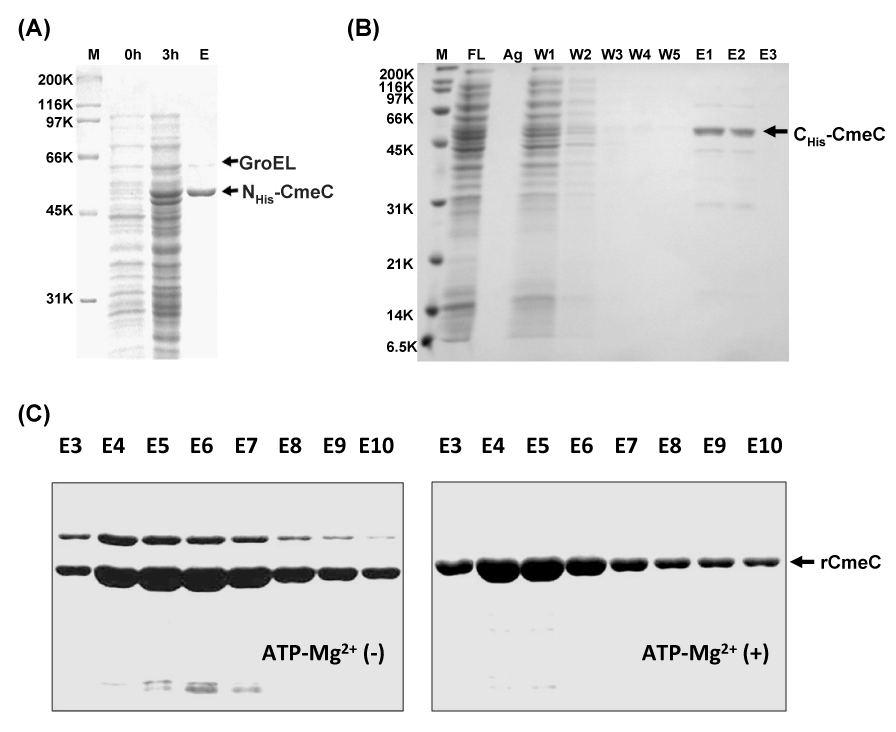
| Figure 3: SDS-PAGE analysis of rCmeC production and purification from E. coli. (A) Expression and purification of N- terminal His-tagged rCmeC. M, standard molecular mass markers (Bio-Rad); 0h and 3h, noninduced and 3 h-induced whole-cell lysate, respectively; E, eluted rCmeC fraction using Ni-NTA affinity chromatography (Qiagen). The putative GroEL, an E. coli molecule chaperone, was consistently co-purified with rCmeC using the standard protocol. (B) Expression and purification of C-terminal Histagged rCmeC. FL: flow through; Ag, Ni-NTA agarose after elution; W1-W5, washing fractions; E1-E3, elution fractions. (C) Efficient removal of GroEL contaminant by ATP-Mg2+ treatment. Eluted fractions (E3 to E10) during Ni- NTA purification with (right panel) or without (left panel) addition of 5 mM of ATP- Mg2+ were subjected to SDS-PAGE analysis. |