 |
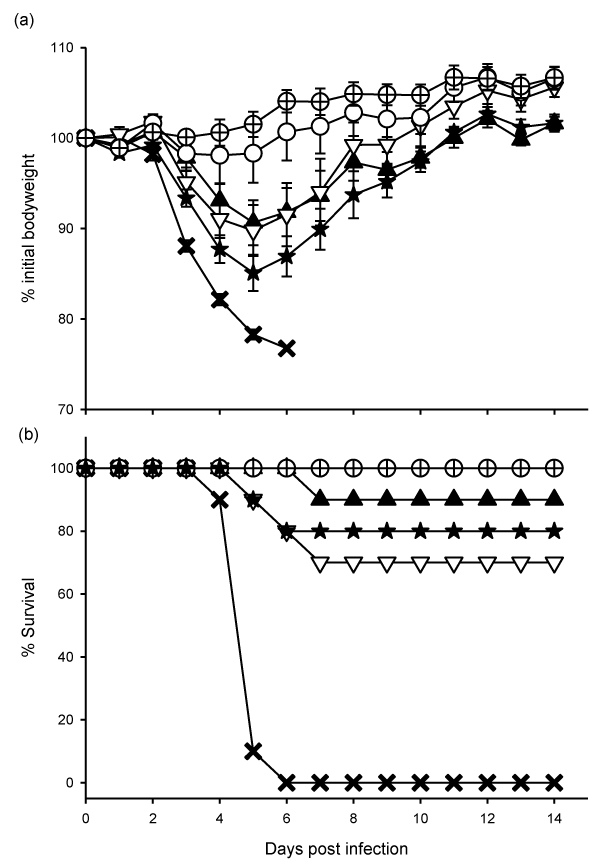
| Figure 2: Clinical manifestations of the adjuvant Trial-1 mice following a lethal dose of influenza virus challenge. Mice (n=10 per group) were intramuscularly injected with a subvirion influenza vaccine (APR8), with or without adjuvant, three times at weekly intervals and were challenged intranasally with 100 MLD50 APR8 two weeks after the third vaccination. Mice were observed daily post challenge for bodyweight (a, mean ± SEM), and survival (b). There were 6 groups of mice, PBS control (?), 10V (?), 1V (?), 1VAl (?), 1V-PIM2 (★) and 1V-Al-PIM2 (⊕). Please refer to Table 1 for vaccine formulations. |