 |
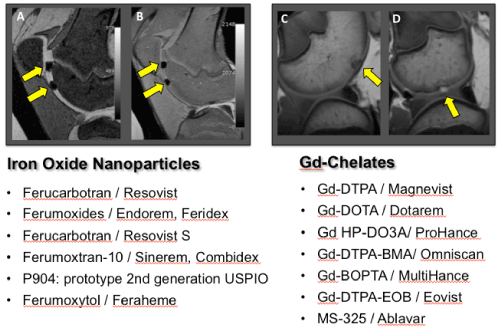
| Figure 2: Overview over clinically applicable MR contrast agents which either have been used for clinical cell tracking studies in the past orwhich could, in principle, be applied “off label” for cell tracking studies in patients, because they are FDA approved for other indications. Sagittal T2-weighted (A) and proton-density-weighted (B) MR scans show example of two transplants of iron oxide nanoparticle labeled cells in cartilage defects (left, arrows) and sagittal T1-weighted MR scans (right) show a representative example of an unlabeled (C) or Gd-DTPA labeled (D) transplant (arrows). |