 |
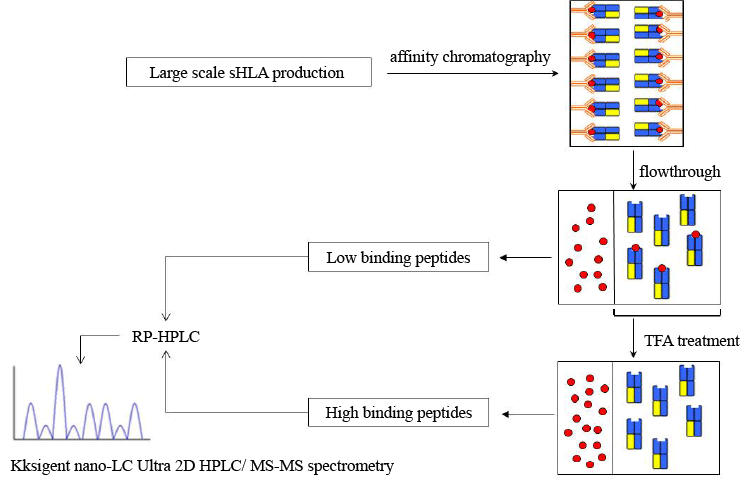
| Figure 3: Engineering soluble HLA-B*44 molecules and peptide sequencing. Schematic overview of the steps involved in the generation of soluble HLA-B*44 molecules. sHLA-B*44 molecules were generated by stable lentiviral transduction of B-LCLs to express these molecules, followed by large scale production of these cells in bioreactors. Supernatant containing sHLA molecules were purified by affinity chromatography using mAb w6/32. Trimeric complexes were eluted using 0.1 M glycine / HCl buffer (pH 2.7). Elution fractions were filtered through a 10 kDa MWCO membrane and the peptides detected in the flow-through were considered to be low binding ones. The retentate containing trimeric complexes were then treated with 0.1 % trifluoroacetic acid (TFA) to elute high binding peptides. Peptides were purified by RP-HPLC and subjected to mass spectrometry using an Eksigent nano-LC Ultra 2D HPLC coupled to an Orbitrap ion trap. |