 |
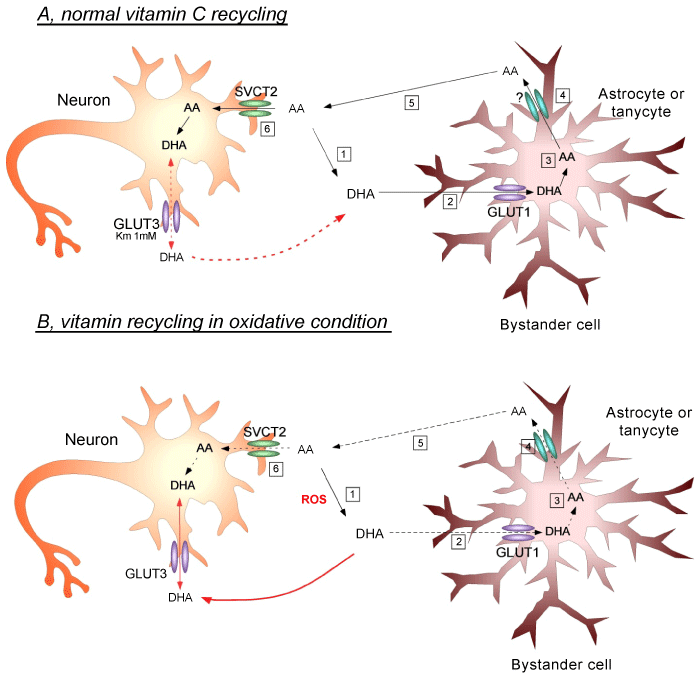
| Figure 5: Vitamin C recycling and proposed interaction between neurons and astrocytes in normal or oxidative stress conditions of the brain. A. Under physiological conditions, reactive oxygen species (ROS) are constantly generated in the CNS. In these conditions, ROS oxidizes AA to DHA (A1), which preferentially enters the astrocyte by GLUT1 (A2). Inside the astrocyte, DHA is again reduced to AA (A3), and is then released into the extracellular space by a yet unknown mechanism (A4). The extracellular AA then enters the neuron via SVCT2 (A5/6), exerting its antioxidant effect and thereby protecting neurons against cell death. Moreover, under normal conditions, we postulate that GLUT3 expression by neurons is involved mainly in DHA efflux. Increased intracellular DHA concentration in the neuron inhibits glycolysis, increases PPP activity, consumes glutathione and increases the influx of lactate. This adaptive mechanism changes the normal metabolism of the neurons in response to the accumulation of DHA [19]. B. In pathophysiological conditions, ROS is massively generated (B1), resulting in increased extracellular DHA concentrations and subsequent DHA entry into astrocytes. However, given the large amount of oxidizing species, the astrocyte will not be able to efficiently reduce DHA to AA (B3), resulting in less AA efflux into the extracellular environment (B4). Subsequently, AA enters the neuron through SVCT2 (B5/6), bringing about a decrease in intracellular antioxidant protection due to its lower degree of uptake by the neuron. Simultaneously, the large extracellular generation of DHA promotes its entry into neurons via GLUT3 (red arrow). Finally, the massive incorporation of intracellular DHA may promote neuronal death by a yet unknown mechanism. |