 |
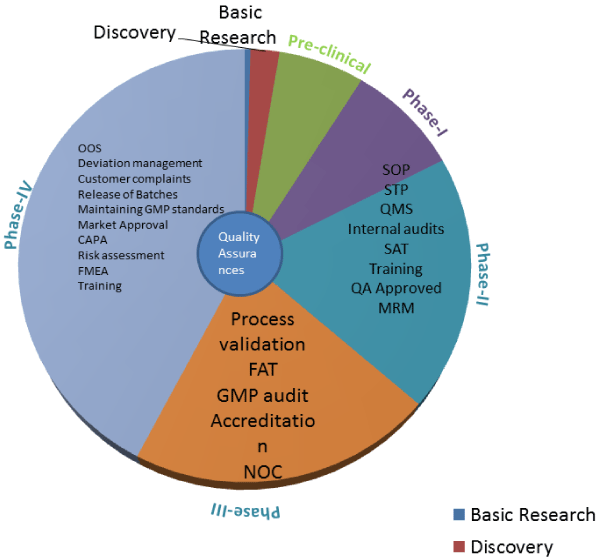
| Figure 5: Roll of Quality Assurance in cell therapy product development-The Quality Assurance Department role in cell therapy product development to post launch of product. Abbreviations: OOS: Out-Of Specification; CAPA: The Corrective and Preventive Action; FMEA: Failure Mode and Effects Analysis; FAT: Field Acceptance Testing; SOP: Standard Operating Procedure; STP: Standard Technical Procedures; QMS: Quality management system; SAT: Site Acceptance Testing; MRM:Management Review Meetings. |