 |
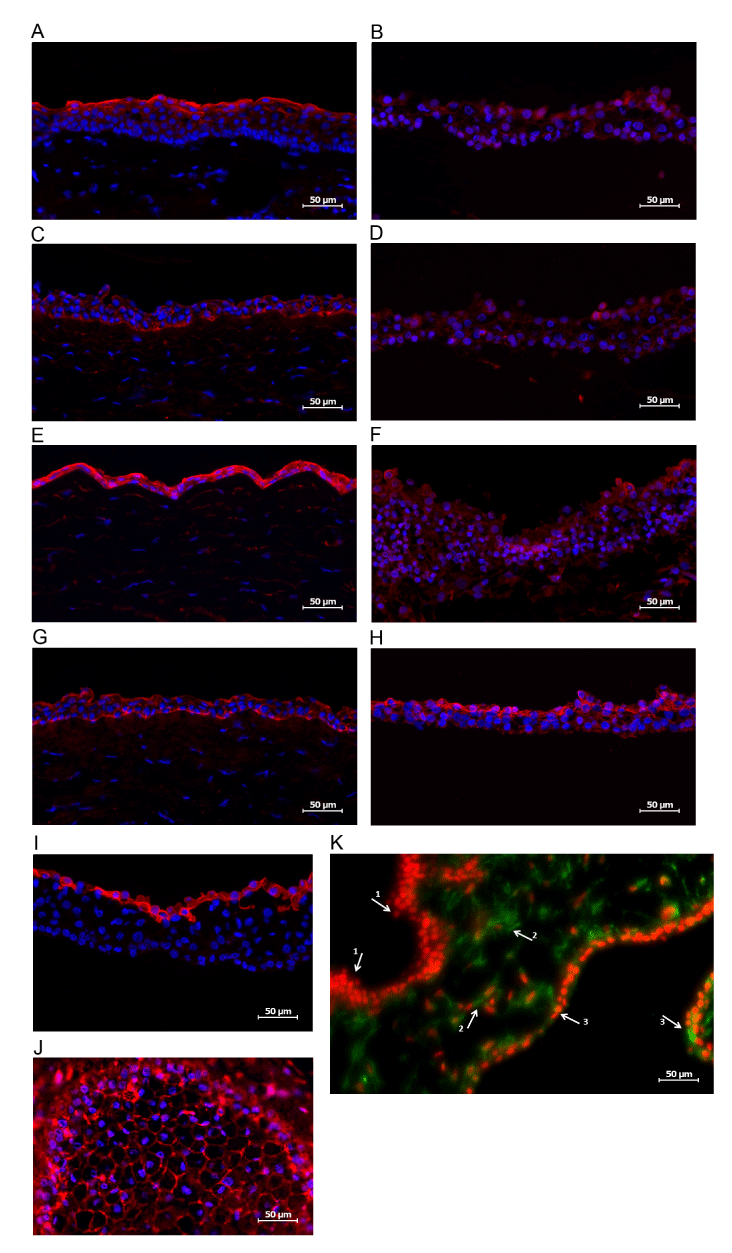
| Figure 3: Fluorescent micrographs of immunostaining performed on corneal orbs at the end of differentiation process. DAPI in A-J marks nuclei in blue, propidium iodide in red (K). All scale bars are 300 μm. A. Mucin-1 immunolstaining (red) of corneal orb concentrated in the epithelial layer is stronger B. Mucin-1 immunostaining (red) of normal adult human donor cornea. C. Connexin-43 immunostaining (red) of corneal orb is concentrated on the innermost layer of epithelial cells and overall staining is more intense than D. Connexin-43 staining (red) of normal adult human cornea. E. Pancytokeratin immunostaining (red) is intense in the epithelial layer of differentiating corneal orbs (confirming patterns in Figure 2). F. Less intense and more diffuse pancytokeratin staining (red) in normal human adult cornea than in differentiating orbs. G. Cytokeratin 19 immunostaining (red) of corneal orbs is intense in the most superficial and deepest epithelial cell layers of the differentiating orbs. H. Cytokeratin 19 immunostaining (red) is most intense in the most superficial epithelial cell lay of normal human adult cornea. I. Cytokeratin 18 (red) is abundant in the most superficial epithelial cell layer of differentiating corneal orbs. J. ZO-1 staining (red) is intense in differentiating corneal orbs with expected subcellular localization to the cell-cell edge interfaces indicating the presence of endothelium. K. Vimentin negative staining (red) of the multiulayered epithelial cells (arrow 1) and the vimentin positive-staining (green) of differentiating corneal orbs is intense in the entire epithelial stromal layer (interspersed stromal keratocytes, arrow 2) and at the predicted anatomic location of the endothelial monolayer (arrow 3). |