| Compound |
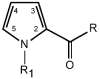 syn
syn |
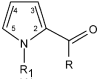 anti anti |
Selected experimental and calculated IR frequencies [cm-1] | ΔEs-cis/s-trans [kcal/mol] | Barrier height Esyn→anti [kcal/mol] |
Barrier height Esyn→anti [kcal/mol] |
HOMA [au] | |||||
| Exp. | Calc.* | Exp. | Calc. | Exp. | Calc. | |||||||
| nN-H | nN-H | nC=O | nC=O | nC=C | nC=C | |||||||
| 1 | pyrole | 3485 | 3600 | 1532b | 1540 | 0.854 | ||||||
| 2 | R1=H, R=H | 3460 | 3565 | 1659d | 1685 | 1552b | 1553 | 3.84 | 16.21 | 10.38 | 0.923 | |
| 3 | R1=H, R=H | 3576 | 1712 | 1554 | 0.911 | |||||||
| 4 | R1=H, R=OH | 3465 | 3570 | 1671d | 1723 | 1555c | 1556 | 1.11 | 11.3 (13.03) |
11.94 | 0.921 | |
| 5 | R1=H, R=OH | 3587 | 1754 | 1561 | 0.916 | |||||||
| 6 | R1=H, R=OCH3 | 3465b 3480b |
3571 | 1719d | 1705 | 1554b 1557c |
1555 | 1.06 (1.10) | 12.35 (12.43) |
11.36 | 0.917 | |
| 7 | R1=H, R=OCH3 | 3589 | 1701d | 1736 | 1559 | 0.913 | ||||||
| 8 | R1=H, R=NH2 | 3415 3336c |
3559 | 1644c 1603c |
1683 | 1552c | 1550 | 4.91 | 10.17 | 6.60 | 0.917 | |
| 9 | R1=H, R=NH2 | 3582 | 1710 | 1560 | 0.882 | |||||||
| 10 | R1=H, R=NHCH3 | 3571 | 1665 | 1558 | 6.02 | 8.75 | 3.33 | 0.914 | ||||
| 11 | R1=H, R=NHCH3 | 3581 | 1690 | 1548 | 0.875 | |||||||
| 12 | R1=H, R=N(CH3)2 | 3450 | 3552 | 1612 | 1623 | 1548a 1547b |
1544 | 4.95 | 7.03 | 2.07 | 0.911 | |
| 13 | R1=H, R2=N(CH3)2 | 3587 | 1697 | 1560 | 0.886 | |||||||
| 14 | R1=H, R=CH2Cl | 3458d | 3562 | 1662d | 1695 | 1546a 1545c | 1546 | 5.15( 3.1) | 13.9 | 10.8 | 0.923 | |
| 15 | R1=H, R=CH2Cl | 3594 | 1683d | 1714 | 1552 | 0.899 | ||||||
| 16 | R1=H, R=CHCl2 | 3457d | 3561 | 1658d | 1692 | 1543a 1542c |
1546 | 4.19 (2.4) | 13.89 | 11.45 | 0.927 | |
| 17 | R1=H, R=CHCl2 | 3562 | 1683d | 1710 | 1548 | 0.921 | ||||||
| 18 | R1=H, R=CCl3 | 3456d | 3563 | 1670b | 1687 | 1538a 1539b 1538c |
1542 | 2.55 (2.51) | 12.85 | 10,33 | 0.926 | |
| 19 | R1=H, R=CCl3 | 3583 | 1679d 1681b |
1708 | 1545 | 0.922 | ||||||
| 20 | methylpyrole | 1517 | 0.865 | |||||||||
| 21 | R1=CH3, R=H | 1670 | 1689 | 1528b | 1531 | 4.52 (3.47) | 15.31 (15.26) | 11.79 | 0.914 | |||
| 22 | R1= CH3, R=H | 1694 | 1533 | 0.906 | ||||||||
| 23 | R1= CH3, R2=OH | 1670 | 1724 | 1530d 1536b |
1531 | 1.93 | 11.19 | 9.25 | 0.913 | |||
| 24 | R1= CH3, R=OH | 1734 | 1536 | 0.904 | ||||||||
| 25 | R1= CH3, R=OCH3 | 1711 | 1706 | 1532b | 1531 | 1.93 | 10.37 | 8.44 | 0.910 | |||
| 26 | R1= CH3, R=OCH3 | 1717 | 1537 | 0.902 | ||||||||
| 27 | R1= CH3, R=NH2 | 1639c | 1683 | 1529c | 1528 | 5.54 | 9.67 | 7.21 | 0.908 | |||
| 28 | R1= CH3, R=NH2 | 1695 | 1537 | 0.882 | ||||||||
| 29 | R1= CH3, R=NHCH3 | 1667 | 1537 | 4.32 | 8.98 | 4.65 | 0.908 | |||||
| 30 | R1= CH3, R=NHCH3 | 1677 | 1542 | 0.877 | ||||||||
| 31 | R1= CH3, R=N(CH3)2 | 1630d | 1635 | 1537a 1536b |
1529 | 3.85 | 13.51 | 0.902 | ||||
| 32 | R1= CH3, R=N(CH3)2 | 1660 | 1542 | 0.870 | ||||||||
| 33 | R1= CH3, R=CH2Cl | 1662d | 1694 | 1527a 1522c |
1526 | 6.61 (5.68) | 12.74 | 6.67 | 0.909 | |||
| 34 | R1= CH3, R=CH2Cl | 1683d | 1693 | 1527 | 0.886 | |||||||
| 35 | R1= CH3, R=CHCl2 | 1658d | 1692 | 1526a 1524c |
1526 | 6.64 (6.97) | 13.5 | 6.59 | 0.905 | |||
| 36 | R1= CH3, R=CHCl2 | 1683d | 1690 | 1527 | 0.889 | |||||||
| 37 | R1= CH3, R=CCl3 | 1690 | 1524a 1522c |
1526 | 8.08 (8.04) | 11.62 | 4.48 | 0.899 | ||||
| 38 | R1= CH3, R=CCl3 | 1681d | 1692 | 1522 | 0.878 | |||||||