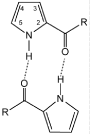
 |
R=H |
R=OH |
R=CH3 |
R=CH2Cl |
R=CHCl2 |
R=CCl3 |
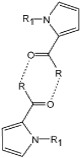 |
R=NH2
R1=H |
R=NH2
R1=CH3 |
R=NHCH3 |
R=OH |
| syn |
anti |
syn |
anti |
syn |
anti |
syn |
anti |
| B3LYP/6-311++G(d,p) |
| N-H |
1.024 |
1.020
(1.019) |
1.022 |
1.022 |
1.022 |
1.019 |
|
1.026 |
1.027 |
|
|
1.01 |
|
1.009
(0.996) |
1.008
(0.99) |
| C=O dim |
1.231 |
1.225
(1.226) |
1.235 |
1.228 |
1.227 |
1.222 |
|
1.243 |
1.235 |
1.241 |
1.238 |
1.251 |
|
1.238
(1.237) |
1.231
(1.230) |
| H…O |
1.835 |
1.88
(1.892) |
1.855 |
1.855 |
1.847 |
1.897 |
|
1.865 |
1.872 |
|
|
1.857 |
|
(1.697) |
(1.688) |
| ∠X-H..O
X= N, O |
164.4 |
163.7
(163.9) |
161.1 |
163.2 |
164.2 |
164.6 |
|
178.6 |
172.7 |
|
|
179.9 |
|
179.7
(178.9) |
180.0
(178.6) |
| ΔvC=O
(vmon-vdim) |
17 |
21 |
|
34 |
28 |
25 |
|
10 |
25 |
|
|
7.5 |
15.6 |
(53) |
(60) |
| EHB
[kcal/mol] |
-6.9 |
-5.9
(-5.7) |
-6.23 |
-6.45 |
-6.63 |
-5.26 |
|
-6.78 |
-6.96 |
-6.01 |
-6.57 |
-6.98 |
-7.61 |
-8.0
(-7.7) |
-8.5
(-8.3) |
| ρBCP
[au] |
0.030 |
0.0263 |
0.0283 |
0.0278 |
0.0282 |
0.0261 |
|
0.0303 |
0.0298 |
|
|
0.0314 |
|
0.0483 |
0.0498 |
| ρRCP
[au] |
0.0029 |
0.0030 |
0.0036 |
0.0036 |
0.0036 |
|
|
0.0048 |
0.0049 |
|
|
0.0058 |
|
0.0081
(0.008) |
0.0081
(0.008) |
| Reference |
[58] |
[39] |
[40] |
[40] |
[40] |
[40] |
|
[69] |
[69] |
[88] |
[88] |
|
|
[39] |
[84] |
|

