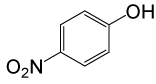
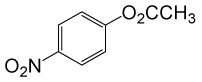
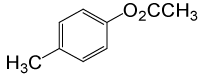
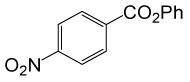
| Entry | Substrate |
Reagent | Product | Time (min) | Yieldb (%) |
| 1 | 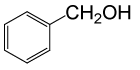 |
(CH3CO)2O | 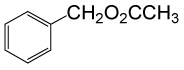 |
80 | 92 |
| 2 | 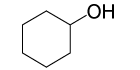 |
4- NO2PhCOCl |
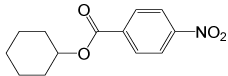 |
95 | 85 |
| 3 | 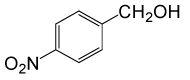 |
(CH3CO)2O | 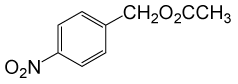 |
80 | 83 |
| 4 | 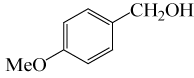 |
(CH3CO)2O | 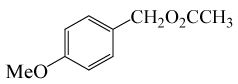 |
90 | 86 |
| 5 | (CH3)2CHOH | 4- NO2PhCOCl | 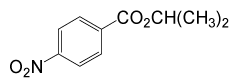 |
95 | 87 |
| 6 | 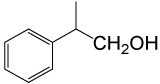 |
(CH3CO)2O | 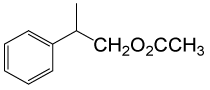 |
85 | 85 |
| 7 | 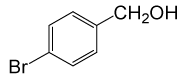 |
(CH3CO)2O | 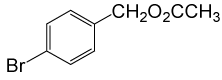 |
80 | 88 |
| 8 | (CH3)2CHOH | 4- CH3PhCOCl | 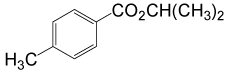 |
90 | 87 |
| 9 | 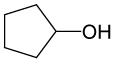 |
PhCOCl | 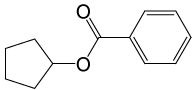 |
100 | 84 |
| 10 | (CH3)3CH2OH | PhCOCl | 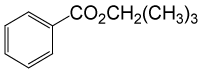 |
105 | 85 |
| 11 | 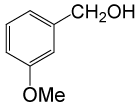 |
(CH3CO)2O | 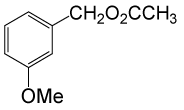 |
95 | 87 |
| 12 | CH3CH2OH | PhCOCl | PhCO2CH2CH3 | 80 | 82 |
| 13 | (CH3)4CH2OH | PhCOCl | 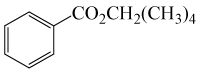 |
95 | 80 |
| 14 | PhCH2OH | PhCOCl | PhCH2O2CPh | 90 | 83 |
| 15 | 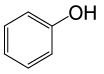 |
(CH3CO)2O | 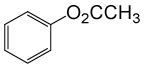 |
110 | 83 |
| 16 |
|
(CH3CO)2O |
|
95 | 89 |
| 17 | 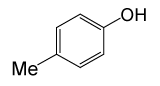 |
(CH3CO)2O |
|
105 | 84 |
| 18 | 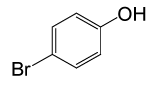 |
(CH3CO)2O | 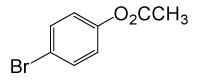 |
100 | 81 |
| 19 | 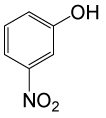 |
(CH3CO)2O | 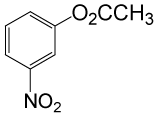 |
90 | 83 |
| 20 | 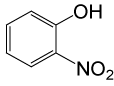 |
(CH3CO)2O | 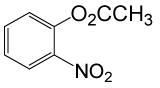 |
95 | 80 |
| 21 | 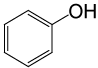 |
PhCOCl |
|
80 | 83 |
| 22 | 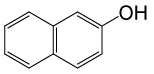 |
(CH3CO)2O | 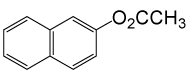 |
100 | 89 |
| 23 | 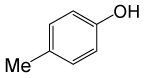 |
PhCOCl | 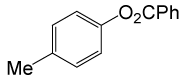 |
110 | 86 |
| 24 | 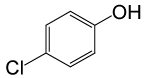 |
(CH3CO)2O | 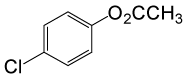 |
95 | 81 |
| 25 | 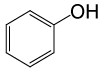 |
PhCOCl | 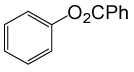 |
105 | 82 |
| 26 | 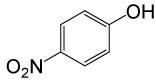 |
4-OMePhCOCl | 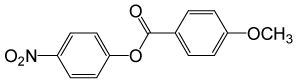 |
110 | 83 |
| 27 | 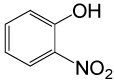 |
4-OMePhCOCl | 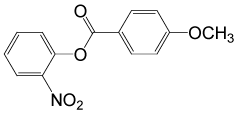 |
95 | 89 |