| Entry |
R1 |
Product |
Yieldb |
| 1 |
4-Me (5a) |
6a |
67% |
| 2 |
5-Me (5b) |
6b |
71% |
| 3 |
6-Me (5c) |
6c |
69% |
| 4 |
7-Me (5d) |
6d |
65% |
| 5 |
5-OMe (5e) |
6e |
80% |
| 6 |
4-F (5f) |
6f |
75% |
| 7 |
5-F (5g) |
6g |
83% |
| 8 |
6-F (5h) |
6h |
82% |
| 9 |
7-F (5i) |
6i |
78% |
| 10 |
5-Cl (5j) |
6j |
85% |
| 11 |
5-Br (5k) |
6k |
84% |
| 12 |
5-I (5l) |
6l |
71% |
| 13 |
5-CN (5m) |
6m |
89% |
| 14 |
5-NO2 (5n) |
6n |
91% |
| 15 |
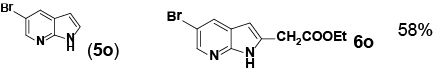 |
| 16 |
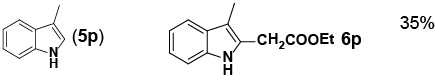 |
| 17 |
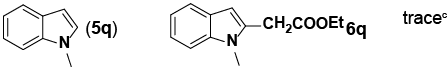 |
aReaction conditions: Pd(PhCN)2Cl2 (0.171 mol), indole (1.71 mmol), norbornene
(3.41 mmol), NaHCO3 (6.84 mmol), ethyl bromoacetate (3.41 mmol), DMF with 0.5
M H2O (8 mL, total), 70°C, 14 h. bIsolated yield. cMonitored by LC-MS |



