 |
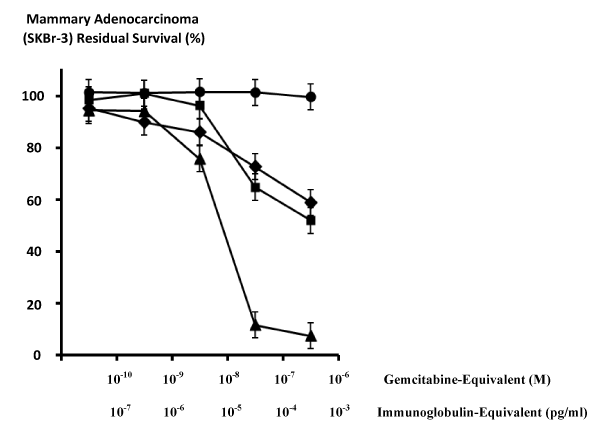
| Figure 4: Differences in cytotoxic anti-neoplastic potency for gemcitabine- (C4-amide)-[anti-HER2/neu], gemcitabine alone, anti-HER2/neu monoclonal immunoglobulin as a function of time. Legends: (♦) covalent gemcitabine- (C4-amide)-[anti-HER2/neu] immunochemotherapeutic following a 182-hours incubation period; (■) gemcitabine chemotherapeutic following a 72-hour incubation period; (▲) gemcitabine chemotherapeutic following a 182-hour incubation period; and (●) anti-HER2/neu monoclonal immunoglobulin Chemotherapeutic-resistant mammary adenocarcinoma (SKBr-3) monolayer populations were incubated with covalent gemcitabine-(C4-amide)-[anti- HER2/neu] and gemcitabine formulated in triplicate at gradient gemcitabineequivalent concentrations. Cytotoxic anti-neoplastic potency was measured using a MTT cell vitality assay relative to matched negative reference controls. |