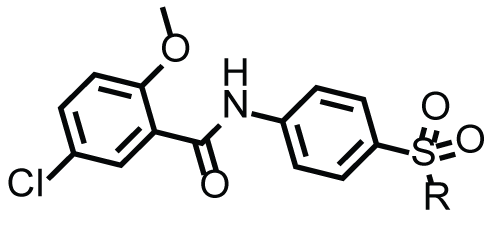
 |
|||
| Compounds | R | Cytotoxicity GI50 (µM)* | |
| A2780 | HCT-116 | ||
| 4a | NHPh | 38.7 ± 3.7 | 48.5 ± 3.0 |
| 4b | NH-(m-Me)Ph | 31.8 ± 5.9 | >100 |
| 4c | NH-(p-Me)Ph | 44.6 ± 3.1 | >100 |
| 4d | NH-(o-Et)Ph | 42.8 ± 4.8 | >100 |
| 4e | NH-(p-Et)Ph | 52.1 ± 27.3 | >100 |
| 4f | NH-(o-CF3)Ph | 45.0 ± 1.3 | >100 |
| 4g | NH-(p-CF3)Ph | 40.6 ± 4.5 | >100 |
| 4h | NH-(o-MeO)Ph | 63.5 ± 6.2 | 76.8 ± 4.7 |
| 4i | NH-(p-MeO)Ph | 46.7 ± 4.6 | 58.1 ± 5.6 |
| 4j | NH-(p-F)Ph | 29.1 ± 3.4 | 39.3 ± 2.9 |
| 4k | NH-(o-Me-4-F)Ph | 42.9 ± 5.8 | >100 |
| 4l | NHCH2Ph | 73.9 ± 5.5 | >100 |
| 4m | NH-iPr | 34.7 ± 2.4 | 59.2 ± 6.9 |
| 4n | NH-nPr | 55.5 ± 16.7 | 69.7 ± 5.2 |
| 4o | NH(CH2)3N(CH3)2 | 31.7 ± 2.5 | 24.7 ± 3.4 |
| 4p | NH-cyclohexyl | 37.6 ± 8.8 | 50.1 ± 4.9 |
| 4q | 1-morpholinyl | 73.8 ± 6.7 | 56.4 ± 5.8 |
| 4r | p-hydroxypipridinyl | 68.7 ± 22.3 | 96.6 ± 10.7 |
| 4s | p-methylpiperazinyl | 45.9 ± 10.7 | 61.6 ± 8.5 |
| 4t | p-acetylpiperazinyl | 48.9 ± 8.2 | >100 |
| E7010 | - | 40.2 ± 2.1 | 64.4 ± 4.3 |