 |
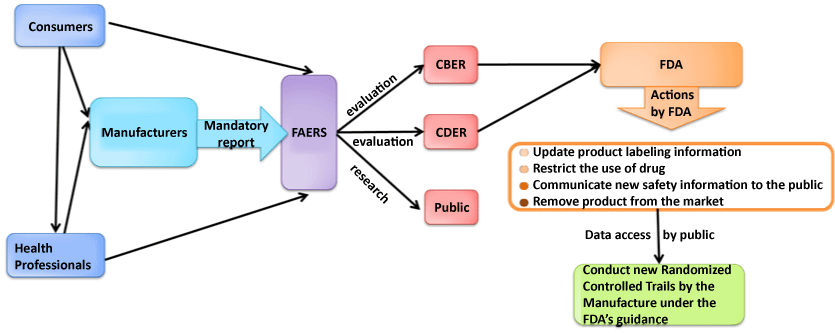
| Figure 2: Pathways of FAERS work flow: The FAERS serves as a central hub for information flow of adverse events report. The early phase communication for suspected ADRs usually occurs among health professionals, individual consumers, and manufacturers. Then, the data are shared and evaluated by the Center for Biologics Evaluation and Research (CBER), Center for Drug Evaluation and Research (CDER), and the public. Eventually, the recommendations and actions from the FDA , which lead to the public awareness are broadcasted to the public. |