 |
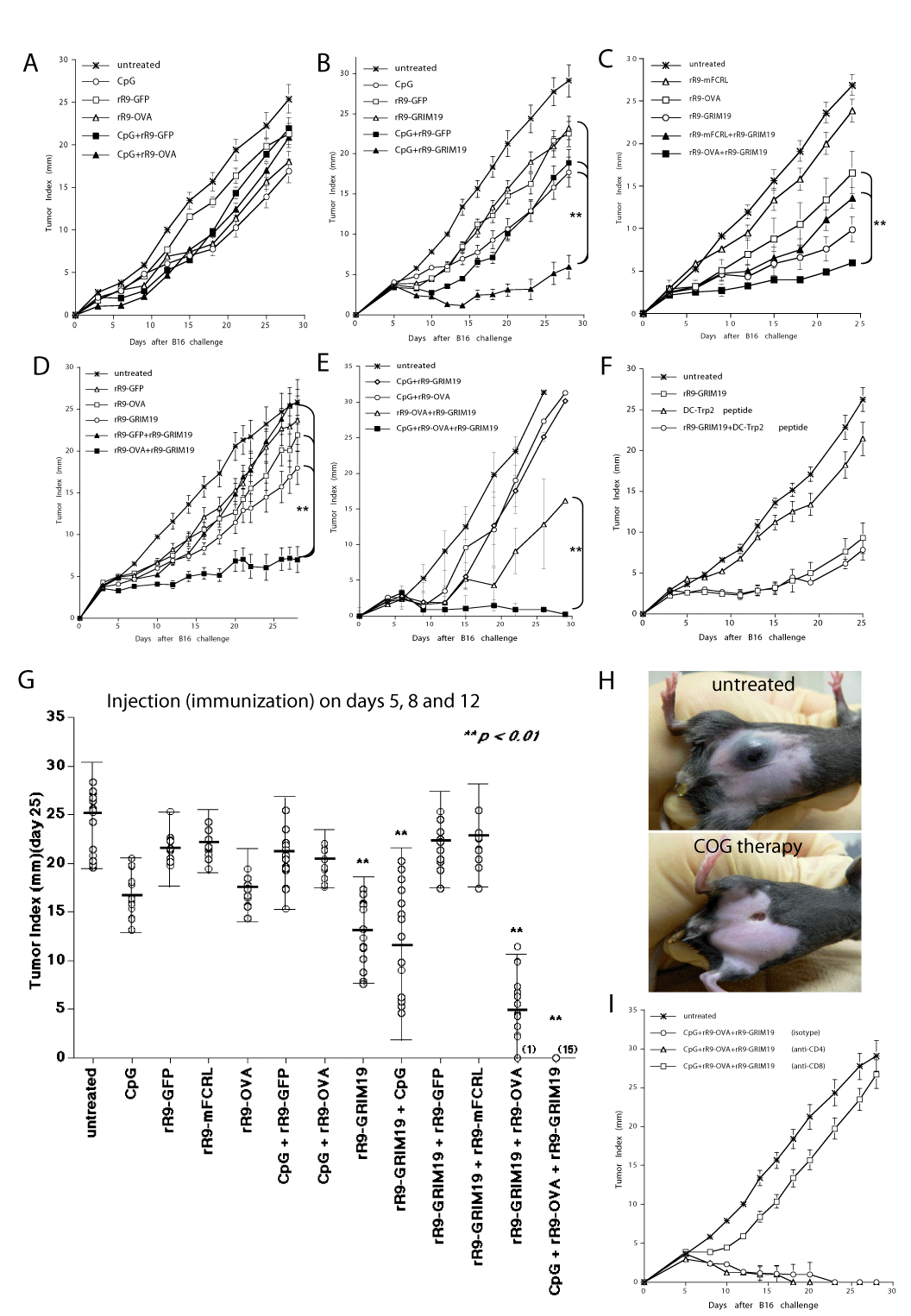
| Figure 3: Enhanced anti-B16 tumor effects by immunotherapies when combined with intratumoral injections of rR9-GRIM19 in vivo. B16 cells (2×105) were subcutaneously injected into naïve mice on day 0. rR9-fusion proteins, CpG, and synthesized Trp2180-188 peptide-pulsed DC were injected intratumorally for 3 days (days 5, 8, and 12) into B16 melanoma-bearing mice. (A-E): Comparison of anti-B16 tumor effects by combination therapy between CpG + rR9-control and CpG + rR9-OVA (A), CpG + rR9-control and CpG + rR9-GRIM19 (B), rR9-mFCRL + rR9-GRIM19 and rR9-OVA + rR9-GRIM19 (C), rR9-GFP + rR9-GRIM19 and rR9-OVA + rR9-GRIM19 (D), or among CpG, rR9-OVA and rR9-GRIM19 (E). (** p < 0.01). (F): Comparison of anti-B16 tumor effects of Trp2-peptide-pulsed-DC therapy with or without rR9-GRIM19. (G): A summary of the anti-B16 tumor effects on day 25 shown in Figure 3A-E. Data are representative of three individual experiments (** p < 0.01). Data from only one representative experiment are shown. (H): Actual B16-bearing site (abdominal skin) after COG therapy (on day 20). (I): Antitumor effects of COG therapy by intraperitoneal injections of depletion anti-CD4, anti-CD8 mAbs or isotype control (500 μg/mouse on days 4 and 11). Data are representative of two individual experiments. Data from only one representative experiment are shown. |