 |
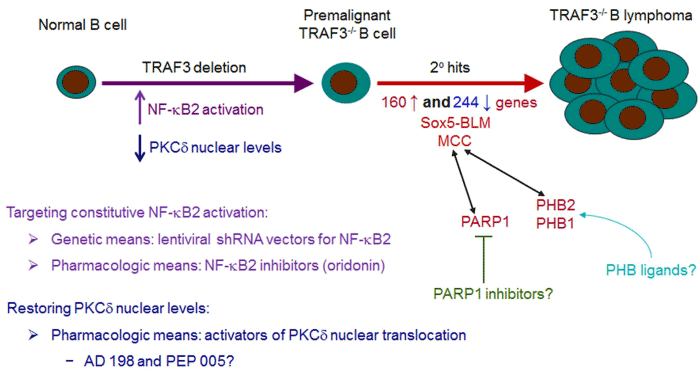
| Figure 2:Targeting TRAF3 downstream signaling pathways in B cell neoplasms. To test whether constitutive NF-κB2 activation can serve as a therapeutic target in B cell neoplasms, we employed genetic and pharmacological means to target the NF-κB2 pathway. Our results demonstrated that lentiviral shRNA vectormediated knockdown of NF-κB2 robustly inhibits the proliferation and induces apoptosis in TRAF3-/- B lymphoma cells. We also found that oridonin, an inhibitor of NF-κB2, exhibits potent anti-tumor activities on TRAF3-/- B lymphomas and human MM. To restore PKCδ nuclear levels, we tested two pharmacological activators of PKCδ nuclear translocation, AD 198 and PEP005. We found that AD 198, but not PEP005, potently kills malignant B cells. However, AD 198 does not induce PKCδ nuclear translocation but specifically targets c-Myc in malignant B cells. Therefore, further studies (using genetic means or more specific PKCδ activators) are required to evaluate the therapeutic potential of activating PKCδ nuclear translocation in B cell neoplasms. Interestingly, TRAF3-/- B cells purified from young B-TRAF3-/- mice exhibit prolonged survival but do not proliferate autonomously, and are therefore premalignant B cells. This suggests that additional oncogenic alterations are required for B lymphomagenesis. To identify such secondary oncogenic alterations, we performed a microarray analysis and identified 160 up-regulated genes and 244 downregulated genes in TRAF3-/- B lymphomas. Among these, we further studied a novel oncogene, MCC, and a new isoform of Sox5, Sox5-BLM, expressed in TRAF3-/- B lymphomas. Since our proteomic data identified PARP1 and PHB2/PHB1 as two signaling hubs of MCC in human MM cells, we are currently testing the therapeutic efficacy of PARP1 inhibitors and PHB ligands in B cell neoplasms with TRAF3 inactivation or aberrant MCC expression. |