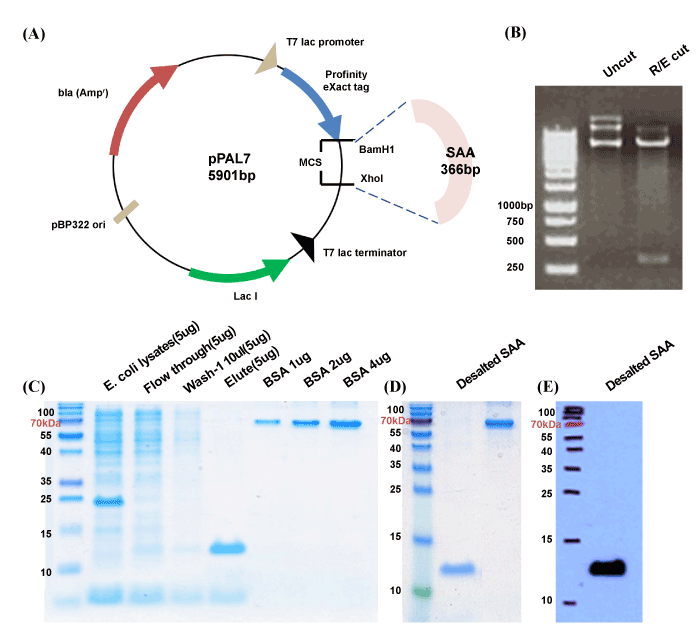
(A) Full length ORF of SAA was cloned into pPAL7 vector by restriction enzymatic digestion. (B) Cloned vector was confirmed by electrophoresis of plasmid cut with two restriction enzymes, BamHI and XhoI, used for the cloning. (C) Commassie Brilliant blue staining of SDS-PAGE shows the results of whole procedures of protein expression and purification process. IPTG treated E.coli lysates showed the major band at the molecular weight of 20 kDa which contains highly expressed SAA (12 k Da) with Profanity eXact tag (8 kDa). Pure SAA without tagging protein was obtained after immunoaffinity purification. SAA proteins were not present in flow through or wash contents during the process. (D) Elutes containing the proteins with low concentration were enriched by 3 kDa-cut off filtrations to 1.5 mg/ml concentration. The concentrated proteins were confirmed by Bradford assay and SDA-PAGE visualized by Coomassie Brilliant Blue (CBB) staining. (E) Desalted SAA was also confirmed by Western blot analysis with commercial SAA polyclonal antibody.