What is the Aftermath of Cerebral Microembolism?
Received: 27-May-2017 / Accepted Date: 12-Sep-2017 / Published Date: 19-Sep-2017 DOI: 10.4172/2161-0460.1000375
Introduction
In Western countries, stroke is the third most common cause of death and the primary cause of permanent disability. Microvascular pathology is common in the aged population, and the incidence of microvascular disease is increasing [1]. Microembolism, which is frequently encountered in patients with atrial fibrillation or cardiovascular surgery, and may partly account for cognitive impairment and dementia in these patients.
Animal models of cerebral ischemia allow for the study of stroke pathophysiology and evaluation of new therapeutic approaches. However, stroke is caused by heterogeneous mechanisms, such as atherothrombosis and embolism, which may result in lacunar, territorial or borderzone infarction. To investigate the pathology of cerebral microembolism, appropriate animal models relevant to human stroke pathology are necessary. Microsphere embolism (ME) in rodents is a useful model for evaluating microvascular injury following microvessel embolism [2-7], since injected microspheres of an appropriate size reach the microcirculation [8].
Brain magnetic resonance imaging (MRI) studies have shown that embolic microinfarction occurs after transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement, with the majority remaining asymptomatic [9,10]. However, these microinfarcts have been shown to be detectable by diffusion-weighted image of MRI in acute phase, or alternatively, by 3D-double inversion recovery (DIR) method even in chronic phase [11], and correlate with patient memory and cognition [12,13]. Indeed, microinfarction may occur concomitantly with cognitive decline in patients undergoing carotid interventions [14]. These observations are underscored by the findings that the rat microsphere embolism model shows the clinical features of vascular dementia [5] as well as human stroke [6]. Therefore, the microsphere embolism model may be a powerful tool to investigate the relationship between cerebral microembolism and impairment of learning and memory.
Since the brain structure of non-human primates is comparable to that of humans, microsphere embolisms in non-human primate brains may show better performance than in the brains of other species [8]. This review focuses on the findings of experimental microembolism and their aftermath in relation to cognitive impairment.
Rodent Models
There are extensive studies on microsphere embolism pathophysiology in the rodent brain. Beley et al. reported cerebral blood flow reduction, increased blood-brain barrier (BBB) permeability, and brain edema 24 h after the injection of 5,000 microspheres (50 μm in diameter) into the rat internal carotid artery (ICA) [2]. Miyake et al. described histological changes in the rat cerebral hemisphere injected with 900 microspheres (47.5 μm in diameter) [4]. After 3 days, the affected hemisphere exhibited scattered necrotic areas that were variable in size and shape and located mainly in the parietotemporal cortex, corpus callosum, hippocampus, thalamus and lenticular nucleus. Moreover, small hemorrhagic foci were found in necrotic areas. Okuyama et al. investigated long-term outcome up to eight weeks after microsphere embolism [3]. They injected 1,000 microspheres (45-54 μm in diameter) into the rat ICA. One to two weeks after embolization, colliquative areas, macrophage or glial cell aggregates, spongiosis and areas of newly formed infarcts were observed in the affected cerebral cortex. In the later period, microspheres were found in the extravascular space, which is indicative of phagocytosis of the occluded arterioles and vascularization. Mayzel-Oreg et al. described neuroimaging findings in rats injected with 1,000 microspheres (50 μm in diameter) into the ICA [6]. Diffusion-weighted spin-echo echo-planar imaging was used to map the average apparent diffusion coefficient (ADCav) of tissue water. Acute-stage ADCav maps and triphenyl tetrazolium chloride staining indicated multifocal and heterogeneous lesion development with slower maturation than that of the middle cerebral artery occlusion model.
Although it was obvious that these microemboli obstructed microvascular structures in the affected hemisphere, their regional distribution and the fate of these particles remained unclear. Zhu et al. traced supraparamagnetic iron oxide-labeled microspheres after injection into the rat [15] and found that 60% of the emboli entered into the penetrating artery. Moreover, the development of ischemic lesions, which varied depending on emboli size and number, was observed. Lam et al. further revealed extravasation of the embolus and recanalization [7]. After injection into the ICA, they traced fluorescently conjugated fibrin clots, cholesterol emboli (8-20 μm in diameter) or polystyrene microspheres (10 or 15 μm in diameter) in green fluorescent protein transgenic mice. Emboli were imaged over time in vivo by transcranial two-photon microscopy. A large fraction of the occluding microemboli failed to be lysed within 48 h. Moreover, some had translocated outside the vessel lumen within 2-7 days leading to the complete reestablishment of blood flow, thereby indicating a novel mechanism of vascular recanalization.
The neurovascular unit comprises the neurons, microvessels and supporting glial cells of the brain. Cerebral microvessels consist of endothelium, basal lamina matrix, pericyte and astrocyte end feet. The intra-arterial infusion of microspheres leads to microvascular injury or BBB disruption, which induces severe brain edema [16]. Han et al. studied ME-induced up-regulation of endothelial nitric oxide synthase (eNOS) in the endothelial cells of brain microvessels [17]. One thousand microspheres (48.4 ± 0.7 μm in diameter) were injected into the left common carotid artery. These authors found ME-induced eNOS expression and protein tyrosine nitration, which occurs predominantly in vascular endothelial cells and results in the production of peroxynitrite and superoxide, and endothelial cell injury. Thus, the upregulation of eNOS in brain microvessels has been shown to account for increased protein tyrosine nitration, thereby disrupting BBB function. These authors also reported that mild (500 microspheres) MEinduced peroxynitrite formation is associated with the accumulation of β-amyloid and phosphorylated tau in the brain microvessels and parenchyma of aged rats [18]. Endothelial cells play important roles in the regulation of β-amyloid clearance in the brain [19]. Therefore, disruption of the β-amyloid clearance system of endothelial cells may allow for β-amyloid accumulation.
Non-Human Primate Models and Human Brain Studies
Non-human primates have similar vessel structures to those of humans and, consequently, are superior models of human stroke. Unfortunately, there are only a few experimental studies of microembolization in nonhuman primates. Macdonald et al. examined microsphere embolisms in the non-human primate brain using histology and MRI and found that emboli entered into the penetrating artery, causing microemboli-induced lacunar infarction [8]. They used 9 cynomolgus monkeys divided into three groups, comprising the Cibacron blue (108,000 ± 7,000 injected beads, 92 ± 28 μm in diameter), Sephacryl (69,000 ± 1,500 injected beads, 68 ± 14 μm in diameter), and Fractogel (1,025,000 ± 54,000 injected beads, 31 ± 4 μm in diameter) groups. For each group, microspheres were injected into the left ICA, and the monkeys were sacrificed 10 min after the injection of the emboli. These authors measured the fraction of the total number of microemboli that entered the penetrating arteries. The percentage of Fractogel, Sephacryl, and Cibacron blue spheres that were observed in penetrating artery territories were 5, 6, and 1.4%, respectively. This study clearly showed that microemboli can enter any cerebral artery that is larger than the embolus, causing lacunar infarction. These authors speculated that embolism also causes microinfarct, which could be clinically undetectable.
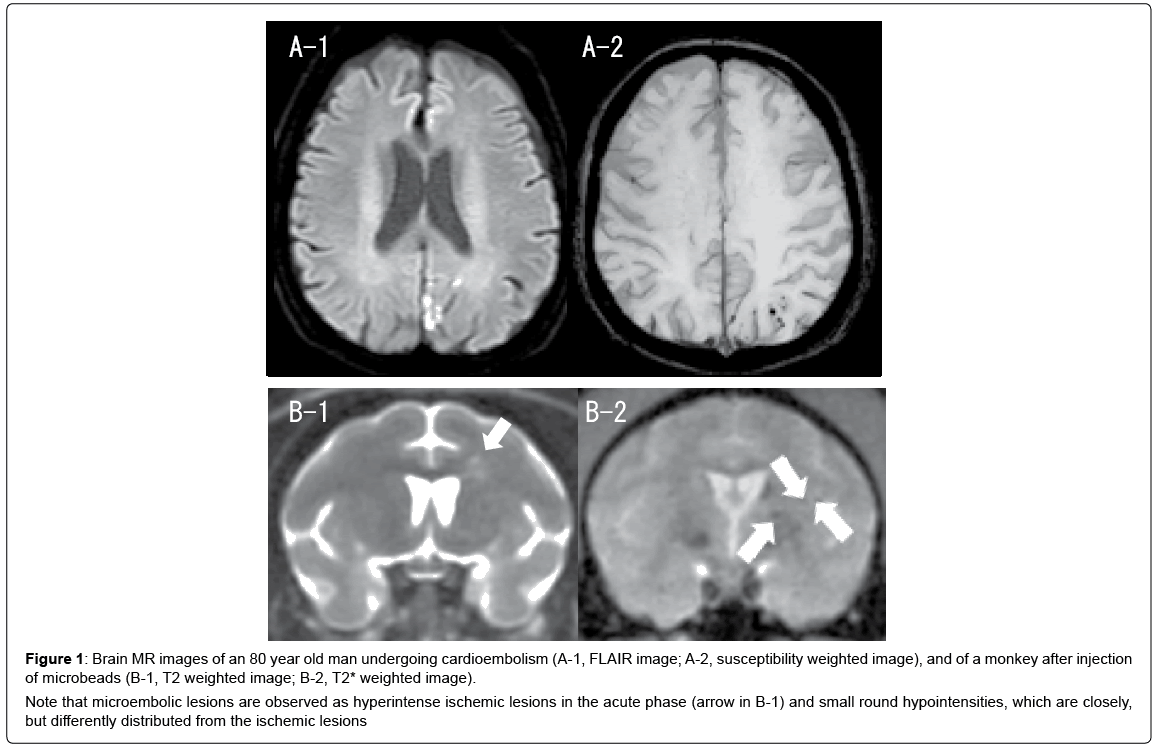
Maki et al. studied brain MRI findings after microsphere embolism in the macaque monkey [20]. Microspheres (Sephacryl; 25-75 μm in diameter, average 50 μm) were injected bilaterally into the ICAs. The number of microspheres injected was 330-2,800 per ICA (660-5,600 in total). The particles were injected into each ICA at a one-month interval. MRI was performed using a 3 Tesla (3T) MRI system, and T1-weighted, T2-weighted, T2*-weighted and fluid-attenuated inversion recovery images were obtained. Multiple microinfarcts were associated with increased particle numbers. In addition to perforating artery infarcts, the monkeys injected with more than 2,000 microspheres per ICA showed cortical and internal watershed infarcts. Moreover, small round hypointensities, which meet the criteria of microbleeds, were detected closely to the ischemic foci in the chronic phase (Figure 1). These results were comparable to human embolic infarction and microbleeds, suggesting that multiple microemboli can cause watershed infarcts and microbleeds both in human and non-human primates.
Figure 1: Brain MR images of an 80 year old man undergoing cardioembolism (A-1, FLAIR image; A-2, susceptibility weighted image), and of a monkey after injection of microbeads (B-1, T2 weighted image; B-2, T2* weighted image).
Note that microembolic lesions are observed as hyperintense ischemic lesions in the acute phase (arrow in B-1) and small round hypointensities, which are closely, but differently distributed from the ischemic lesions
It has been demonstrated that microembolism causes watershed infarcts in the human brain. Pollanen et al. studied the brains of cadavers to examine the mechanisms of embolic watershed infarcts [21]. They J Alzheimers Dis Parkinsonism, an open access journal Volume 7 Issue 5 • 1000375 ISSN:2161-0460 perfused the brains of cadavers with suspensions of 90-210 μm glass microspheres and chemically extracted the particles from various arterial territories and a watershed zone. Particles that were 150-210 μm in size were preferentially distributed in the watershed zone. The selective distribution of emboli, which cause watershed infarcts, has also been reproduced in patients that underwent atrial fibrillation transcatheter ablation [22].
Discussion
Rodent and non-human primate models have demonstrated that microemboli enter into the penetrating artery. Microembolism can cause lacunar infarction and microinfarction. BBB dysfunction following microembolization has been extensively studied. Intraarterial injection of microspheres leads to microvascular injury and BBB disruption, inducing severe brain edema. Additionally, microembolism induces the up-regulation of eNOS in endothelial cells. Peroxynitrite formation by eNOS is associated with endothelial cell injury, which disrupts BBB function. Impaired BBB function may affect the β-amyloid clearance system of endothelial cells in the aged rats [18]. A recent study further revealed that microinfarction induces decrease of aquaporin 4 and dysfunction of glymphatic system which is closely associated β-amyloid clearance [23]. This mechanism may be associated with the pathophysiology of Alzheimer’s disease. Alternatively, microemboli may impair directly neuronal function by way of ischemic damages [24].
In human pathological studies, cerebral microbleeds occurred at the small artery, arteriole and capillary level. Fisher stresses the heterogeneity of microbleeds and proposes their classification as primary, secondary, or pseudo-microbleeds, depending on their pathologic mechanisms [25]. Primary microbleeds are caused by arterial rupture or disruption of the BBB, whereas secondary microbleeds include hemorrhagic infarction and microinfarction. In particular, microembolism may lead to hemorrhagic (micro) infarction or alternatively, embolus extravasation at the capillary level, which has been observed after embolization in rodent models [26]. Clearly, further studies are needed to elucidate the aftermath of microembolism, including infarction, microinfarction and microbleeds. In particular, it is noteworthy that cerebral microbleeds are not necessarily caused by small vessel disease, but at least a part of cerebral microbleeds are caused by microembolism which should be termed as “embolic microbleeds”. Investigation of the pathological counterpart of microbleeds may further clarify the significance of microbleeds in non-human primate microembolic models.
Conclusion
In conclusion, the experimental microembolism model is a powerful tool for evaluating embolic stroke and microvascular injuries in patients with atrial fibrillation and cardiovascular surgery.
References
- Chen RL, Balami JS, Esiri MM, Chen LK, Buchan AM (2010) Ischemic stroke in the elderly: An overview of evidence. Nat Rev Neurol 6: 256-265.
- Beley A, Edvinsson L, Hardebo JE (1981) Cerebral microembolization in the rat: Changes in blood-brain barrier permeability and cerebral blood flow as related to the degree of ischemia. Acta Neurol Scand 64: 88-100.
- Okuyama K, Kiuchi S, Okamoto M, Iwasaki H, Narita H, et al. (1998) Time-dependent changes in the ischemic forebrain following the microsphere-induced permanent occlusion of cerebral arterioles in rats. Jpn J Pharmacol 78: 31-37.
- Miyake K, Takeo S, Kaijihara H (1993) Sustained decrease in brain regional blood flow after microsphere embolism in rats. Stroke 24: 415-420.
- Takagi N, Miyake K, Taguchi T, Sugita N, Takagi K, et al. (1997) Changes in cholinergic neurons and failure in learning function after microsphere embolism-induced cerebral ischemia. Brain Res Bull 43: 87-92.
- Mayzel-Oreg O, Omae T, Kazemi M, Li F, Fisher M, et al. (2004) Microsphere-induced embolic stroke: an MRI study. Magn Reson Med 51: 1232-1238.
- Lam CK, Yoo T, Hiner B, Liu Z, Grutzendler J (2010) Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature 465: 478-482.
- Macdonald RL, Kowalczuk A, Johns L (1995) Emboli enter penetrating arteries of monkey brain in relation to their size. Stroke 26: 1247-1250.
- Kahlert P, Knipp SC, Schlamann M, Thielmann M, Al-Rashid F, et al. (2010) Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: A diffusion-weighted magnetic resonance imaging study. Circulation 121: 870-878.
- Uddin A, Fairbairn TA, Djoukhader IK, Igra M, Kidambi A, et al. (2015) Consequence of cerebral embolism after transcatheter aortic valve implantation compared with contemporary surgical aortic valve replacement: Effect on health-related quality of life. Circ Cardiovasc Interv 8: e001913.
- Ii Y, Maeda M, Kida H, Matsuo K, Shindo A, et al. (2013) In vivo detection of cortical microinfarcts on ultrahigh-field MRI. J Neuroimaging 23: 28-32.
- Blum S, Luchsinger JA, Manly JJ, Schupf N, Stern Y, et al. (2012) Memory after silent stroke: hippocampus and infarcts both matter. Neurology 78: 38-46.
- Ueda Y, Satoh M, Tabei K, Kida H, Ii Y, et al. (2016) Neuropsychological features of microbleeds and cortical microinfarct detected by high resolution magnetic resonance imaging. J Alzheimers Dis 53: 315-325.
- Hitchner E, Baughman BD, Soman S, Long B, Rosen A, et al. (2016) Microembolization is associated with transient cognitive decline in patients undergoing carotid interventions. J Vasc Surg 64: 1719-1725.
- Zhu L, Hoffmann A, Wintermark M, Pan X, Tu R, et al. (2012) Do microemboli reach the brain penetrating arteries? J Surg Res 176: 679-683.
- Kogure K, Busto R, Scheinberg P, Reinmuth OM (1974) Energy metabolites and water content in rat brain during the early stage of development of cerebral infarction. Brain 97: 103-114.
- Han F, Shirasaki Y, Fukunaga K (2006) Microsphere embolism-induced endothelial nitric oxide synthase expression mediates disruption of the blood-brain barrier in rat brain. J Neurochem 99: 97-106.
- Han F, Ali Raie A, Shioda N, Qin ZH, Fukunaga K (2008) Accumulation of beta-amyloid in the brain microvessels accompanies increased hyperphosphorylated tau proteins following microsphere embolism in aged rats. Neuroscience 153: 414-427.
- Deane R, Bell RD, Sagare A, Zlokovic BV (2009) Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer's disease. CNS Neurol Disord Drug Targets 8: 16-30.
- Maki T, Wakita H, Mase M, Itagaki I, Saito N, et al. (2011) Watershed infarcts in a multiple microembolic model of monkey. Neurosci Lett 499: 80-83.
- Pollanen MS, Deck JH (1990) The mechanism of embolic watershed infarction: Experimental studies. Can J Neurol Sci 17: 395-398.
- Bergui M, Castagno D, D'Agata F, Cicerale A, Anselmino M, et al. (2015) Selective vulnerability of cortical border zone to microembolic infarct. Stroke 46: 1864-1869
- Venkat P, Chopp M, Zacharek A, Cui C, Zhang L, et al. (2017) White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiol Aging 50: 96-106.
- Zhang HA, Gao M, Chen B, Shi L, Wang Q, et al. (2013) Evaluation of hippocampal injury and cognitive function induced by embolization in the rat brain. Anat Rec (Hoboken) 296: 1207-1214.
- Fisher M (2014) Cerebral microbleeds: where are we now? Neurology 83: 1304-1305.
- Fisher M, French S, Ji P, Kim RC (2010) Cerebral microbleeds in the elderly: A pathological analysis. Stroke 41: 2782-2785.
Citation: Wakita H, Shindo A, Tomimoto H (2017) What is the Aftermath of Cerebral Microembolism? J Alzheimers Dis Parkinsonism 7: 375. DOI: 10.4172/2161-0460.1000375
Copyright: © 2017 Wakita H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4749
- [From(publication date): 0-2017 - Apr 19, 2024]
- Breakdown by view type
- HTML page views: 4133
- PDF downloads: 616