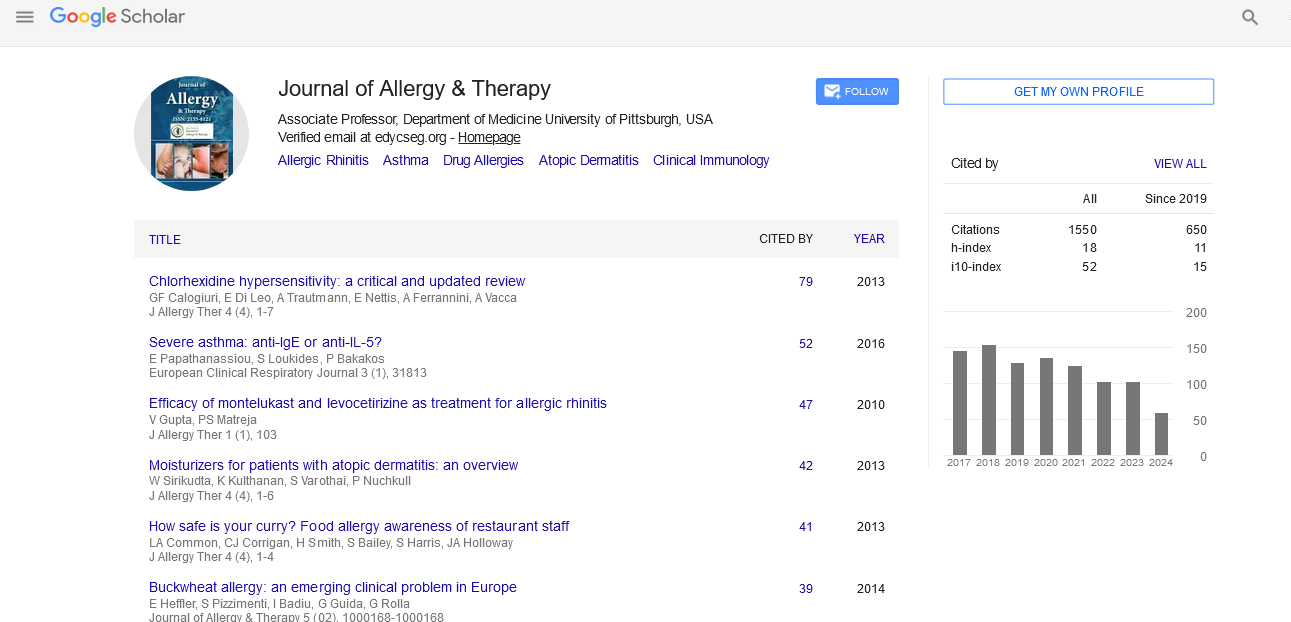
PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
p21 is a master regulator of NF-�úB-dependent inflammation in immune disease and allergic asthma
11th International Conference on ALLERGY, ASTHMA & CLINICAL IMMUNOLOGY
September 07-08, 2017 | Edinburgh, Scotland
Dimitrios Balomenos
National Center for Biotechnology - CSIC, Spain
Scientific Tracks Abstracts: J Allergy Ther
Abstract:
Decontrolled inflammatory responses induce live-threatening disease or aggravate autoimmunity or allergy. p21 was first identified as an inhibitor and cyclin-dependent kinase 2 inhibitor (CDK2). However, other functions are now attributed to p21, as it orchestrates unique anti-inflammatory functions. p21 controls NF-�?ºB activation, macrophage activation and LPS-induced septic shock. In the absence of p21, LPS treatment leads to increased NF-�?ºB activation, macrophage overstimulation and excessive inflammatory production. Upon extreme TLR4 activation, macrophage hyper activation is also controlled by reprograming of hyperinflammatory microphages (M1) to a hypo responsive status (M2-like) after a secondary LPS challenge, which is known as endotoxin tolerance. We recently reported that p21 promotes M1-to-M2 polarization, as p21-/- macrophages showed impaired ability to reprogram. p21 shifts the balance between active p65/p50 and inhibitory p50/p50 NF-�?ºB and promotes macrophage reprogramming by favoring DNAbinding affinity of p50/p50 homodimers, which limit IFN-�?¥ production. The first association of p21 with the immune response was its identification as an autoimmunity suppressor, and it was assumed to control T cell proliferation. However, our latest findings show that lack of p21 drives lupus-like disease by augmenting the activation status of T cells. The major characteristic of this hype activation is the elevated production of IFN-gamma by T cells, a major inducer of lupus autoimmunity. Our findings point to p21 as an early regulator of T cell activation that controls mitochondrial ROS production. Finally, in agreement with effect of p21 on the excessive immune responses, p21-deficient mice developed extreme allergic asthma when subjected to the allergy-inducing OVA model, exhibiting severe pneumonitis and striking perivascular and interstitial inflammation compare to controls. Overall p21 emerges as a global guardian of hyperresponsivess in inflammatory, autoimmune and allergic pathologies at both the macrophage and T cell levels.
Biography :
Dimitrios Balomenos received his PhD of Rutgers University, NJ. and continued his postdoctoral training at the Scripps Research Institute (La Jolla, Ca) and in the Department of Immunology and Oncology of CNB/CSIC in Madrid, Spain. In 2002 he was awarded an independent group research contract and from 2010 he is a tenured group leader at CNB/CSIC. Major discoveries include the absolute requirement of IFN-gamma for Systemic Lupus Erythematosus pathogenesis, (D. Balomenos et al, J. Clin. Invest. 1997), the discovery of p21-dependent pathways for regulation of T cell tolerance and autoimmunity (D. Balomenos et al, Nat. Med. 2000), and the requirement for p21 in reprogramming of M1-to-M2 macrophages, though the regulation of p50/p50 NF-kB and IFN-beta expression (Rackov et al, 2016). The group focuses in understanding how p21 and other molecules restrain extreme immune responses and discover p21 molecular patners that control ecssessive infalmmation and might harbor potential treatment capacity.