| Research Article |
Open Access |
|
| Mahboubeh Hosseinzadeh, Mohammad Reza Imanpoor and Hamed Nekoubin* |
| Department of Fishery, Gorgan University of Agricultural Sciences and Natural Resources, Gorgan, Iran |
| *Corresponding author: |
Nekoubin H
Department of Fishery
Gorgan University of Agricultural Sciences and Natural Resources
Gorgan, Iran
E-mail: nekoubin.hs@gmail.com |
|
| Â |
| Received August 12, 2012; Published November 05, 2012 |
| Â |
| Citation: Hosseinzadeh M, Imanpoor MR, Nekoubin H (2012) Histology of Ovarian Development and Maturity Stages in the Wild Persian Sturgeon, Acipenser persicus. 1:483. doi:10.4172/scientificreports.483 |
| Â |
| Copyright: © 2012 Hosseinzadeh M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| The Persian sturgeon, Acipenser persicus, is a vulnerable anadromous fish considered to biological conservation program in southern part of Caspian Sea. Correct classification of ovaries into a maturity condition is necessary for accurate estimation of maturity. We investigated 35 specimens female Persian sturgeon. In present study four developmental stages of ovary including cortical alveoli formation stage (ΙΙ), Vitellogenic stage (ΙII), mature stage (IV) and Post-spawningare recognized during development. Maturity condition was assessed by gonado somatic index (GSI), and histological methods. The GSI of female Persian sturgeon was gradually increased during the development of ovary. Also, GSI of this fish in present study correlated with development of gonad. In generally, GSI may be useful to determine maturity stages; however histological examination of ovaries is the most accurate method for all stages. |
| Â |
| Keywords |
| Â |
| Acipenser persicus; Histological; Gonado somatic index; Maturity stages; Caspian sea |
| Â |
| Introduction |
| Â |
| Histology studies currently in many biological phenomena such as fish reproduction to invent new and effective methods for increasing efficiency of bloodstock, increasing fish production and ultimately increase efficiency and higher fish are predicted. Determine the peak period of spawning assessment and exploitation of fish, understanding the biological characteristics and life cycle of a species also supplies management and reconstruction is an important role [1]. |
| Â |
| Successful management of sturgeon populations requires knowledge of the stock composition with regard to sex and maturational status [2]. |
| Â |
| Sexual maturation is delayed in sturgeons and paddlefishes. Gametogenesis and gonadal cycles were elucidated in cultured Acipenser baerii [3], the hybrid Huso huso × A.ruthenus [1,4] A. schrenckii [5], and A. transmontanus [6]. |
| Â |
| When the primary oocyte enters the diplotene stage of the first meiotic division, karyokinesis is arrested and cell division halts until the final stages of sexual maturation. The primary oocyte then undergoes a series of changes to a vitellogenic oocyte, a secondary oocyte, and finally a fully matured ovulated oocyte. The growth from oogonium to mature oocyte is considerable. This increase in size is attributable mainly to accumulation of yolk proteins. The gonadosomatic index of the ovary increases in concert with increasing size of the oocytes [7]. |
| Â |
| The Persian sturgeon, Acipenser persicus, is a vulnerable anadromous fish considered to biological conservation program in southern part of Caspian Sea [8]. |
| Â |
| Sexual maturity in sturgeon fish is reached under natural conditions after 8 to 13 years in males and after 10 to 16 years in females [9]. However, under aquaculture conditions, sturgeon maturity is usually reached at an earlier age [6]. |
| Â |
| In older females approaching puberty, information on ovarian developmental stage is critical for the quality of caviar production. It should be borne in mind that vitellogenesis in cultured sturgeons can last up to 3 or 4 years with large variations among individual fish of the same age [4,6]. Therefore, estimating the optimal time for caviar harvest requires several examinations of each individual female [10]. |
| Â |
| Since the complexity of most biological problems to obtain oocytes and oocytes for the required artificial amplification and is defined manufacturing processes more oocytes consider the knowledge of biology and ovarian development is especially important. Sturgeons are not obviously sexually dimorphic; therefore, sex and stage of maturity are currently determined through gonadal biopsy [2]. |
| Â |
| Therefore, this study investigated growth and ovarian development of Persian sturgeon in the Caspian Sea area. Histology studies are able to provide the necessary and appropriate strategies for optimum utilization and maintain supplies of this valuable species harvested step. |
| Â |
| Materials and Methods |
| Â |
| In this study, 35 specimens female Persian sturgeon (A. persicus) was captured from southeast of Caspian Sea during a year. Total weight and fork length of the fishes were measured. The fish, after they had been sacrificed. The gonad removed weighted (kg) and examined microscopically Pieces of gonads were cut and the gonad samples were fixed in Bouin's fluid for 48 h and then transferred to 70% ethanol for storge until processing for light microscopy. Paraffin sections of 4–7 μm were stained with hematoxylin and eosin. |
| Â |
| The captured fishes in late winter and early spring that were in stage III-IV or IV transferred to Shahid Marjani sturgeon Propagation Center in Gorgan, Iran. Two injections of sturgeon pituitary preparation (PP) (3-5 mg/kg) were used to simulate final maturation (stage V). The first injection PP (5% of total dose) was made at 10 pm and second (95% of total dose) 12 h later at 8 am hours. And 24 h after second injection the gonad removed weighted (kg) and examined microscopically. |
| Â |
| Sexual maturity was determined by examining the sections under a light microscope. We classified the gonad developmental stages according to the system [1,4]. |
| Â |
| The gonadosomatic index (GSI) of female fish was calculated by dividing the ovaries weight (WG) by the whole body weight (WT) and multiplying by 100 [11]. |
| Â |
| GSI=WG/WT×100 |
| Â |
| Results |
| Â |
| Total weight (27.11 ± 5.45 kg) and fork length (153.72 ± 19 cm) of the fishes were measured. |
| Â |
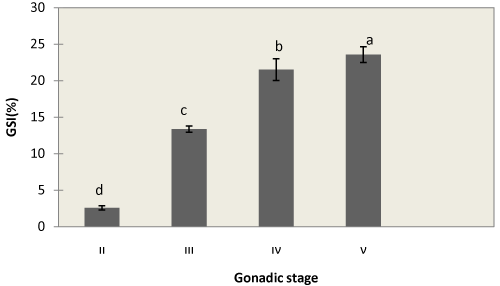
| GSI in stage II was very small (2.57 ± 0.28%) and GSI in stage III and IV increased and reached 13.37 ± 0.42% and 21.51 ± 1.49%, respectively. And it was maximum in stage V (23.58 ± 1.08%) (Figure 1). |
| Â |
|
|
Figure 1: Changes in GSI correlated with Gonad development of A. persicus. Each value is mean ± SD. Means with different latter subscripts are significantly different (P<0.05). |
|
| Â |
| Four developmental stages of ovary of A. persicus are recognized during development. |
| |
| Â |
| 1. Cortical alveoli formation stage (ΙΙ) |
| Â |
| 2. Vitellogenic stage (ΙII) |
| Â |
| 3. Mature stage (IV) |
| Â |
| 4. Post-spawning (v) |
| Â |
| The observation via light microscopy in this study revealed different histological structure of each oocyte developmental stage. |
| Â |
| Stage II: this stage is characterized by the appearance of clear vesicles in the cytoplasm. The vesicle was begun to accumulate from the periphery of the oocyte. The nuclei were still perinucleolar. In this stage, a thin acidophilic zonaradiata or primary envelope became visible for the first time. Follicular layers were also seen at the first time to consist of simple cuboidal or columnar layer surrounded with stratified squamous thecal layer (Figure 2A). |
| Â |
|
|
Figure 2: Histological sections of A. persicus. Cortical alveoli formation stage (A), Vitellogenic (yolk) stage (B), mature stage (C). Post-spawning (D). ad: adipose tissue, pvo: previtellogenic oocyts, n: nucleus, yp: yolk platelets. |
|
| Â |
| Stage III: the oocyte size increased. Small yolk granules were visible as a ring of deep eosinophilic in the cytoplasm and later incorporated the whole cytoplasmic area. The nucleus was still convoluted. The zona radiata was clearly visible as a noncellular deep eosinophilic band. Follicular layers were well- developed simple cuboidal or columnar layer surrounded by stratified squamous thecal layer (Figure 2B). |
| Â |
| Stage IV: in this phase of development, vitellogenesis has reached its peak, the cell has become larger and more hydrated, and the cleusnu has migrated toward the periphery of the cell and is in the process of dissolution (Figure 2C). |
| Â |
| Stage V: oocytes were characterized by large mass of yolk. eggs have hydrated and the appearance of flowing sexual products is noted, commencement of spawning is ready to begin. histologically, large size oocytes with coarse yolk granules scattered in the cytoplasm are presents (Figure 2D). |
| Â |
| Discussion |
| Â |
| Female reproductive maturity was commonly quantified by the GSI (Lowe-McConnell, 1982) [12]. Our results show that the GSI of female Persian sturgeon was increased during the development of ovary. According to the seasonal variation of GSI, the gonads start to develop in summer and mature in spring, when the highest values of GSI (21.51 ± 1.49%) were recorded. |
| Â |
| Accurately, the gonadal development of fish begins at as early as gonadal origin, or formation of primary germinal tissue. This early stage of gonadal development, as well as sex differentiation of gonads has been poorly investigated in Acipenseriforms [13]. Female reproductive maturity was commonly quantified by the GSI [12]. Our results show that the GSI of female Persian sturgeon was increased during the development of ovary. Also, GSI of this fish in present study was correlated with development of gonad. |
| Â |
| However, determination of reproductive maturity using only the GSI is not enough because the structures within the ovary such as oocytes at different stages, interstitial tissue with accumulation of yolk materials, con not be interpreted by weight. Direct observation of histological architecture is the most accurate method to let us know exactly the stage of maturation at the ovary is undergoing [14]. |
| Â |
| The present study on ovarian histology of A. persicus revealed the basic histological architecture and identified the oocytes found within the ovary. It provides a basic knowledge for other studies such as reproductive biology. |
| Â |
| Histological changes in ovary during the reproductive cycle were similar in another species. Briefly Oogonia proliferate through mitotic division of primary germ cells, and transform into previtellogenic oocytes, characteristic of the immature ovary. The elaboration of yolk in the oocyte marks the beginning of vitellogenesis at the end of which the cell attains its maximum size and undergoes maturation/ovulation, followed by the extrusion of egg to the exterior Seedo [15]. |
| Â |
| Female gametogenesis comprises several developmental steps and consists of oogenesis, oocyte growth, maturation, and ovulation. Primordial germcells differentiate into oogonia under the influence of early gene cascades and steroid signaling. Oocyte growth is triggered by gonadotropins, and the subsequent steroidogenic production of estradiol induces vitellogenesis, leading to a marked enlargement in oocyte size. Neuroendocrine factors including stimulatory and inhibitory signals are regarded as major regulators of oocyte development and mediators of environmental and physiological cues. Maturation requires meiotic resumption, and is triggered both by gonadotropins and maturation-inducing hormone. Gonadotropin signals are mediated or modulated in the ovary by a complex local paracrine network of peptide factors [16]. |
| Â |
| The rhythm of deposition of yolk inclusions in the oocyte of fish differs from species to species. In Tilapia mossambica, lipid vesicles and yolk granules appear in the oocyte at the same time [17]. In other teleosts, lipid vesicles are the first type of yolk inclusion to appear in vitellogenic oocytes, their appearance marking the onest of vitellogenesis [18,19]. The results of the present study are in agreement with those of latter researchers, that lipid yolk appears first in oocyte before the elaboration of yolk granules as the second type of yolk inclusion. |
| Â |
| In generally, regarding the seasonal variation in gonad maturity stage, the breeding season of Persian sturgeon take place in late winter and spring, between March and May [20]. |
| Â |
| Acknowledgment |
| Â |
| The authors are grateful to Shahid Marjani sturgeon Propagation Center in Gorgan, Iran, for helping our study. |
| Â |
| |
| References |
| Â |
- Amiri BM, Maebayashi M, Hara A, Adachi S, Yamauchi K (1996) Ovarian development and serum sex steroid and vitellogenin profiles in the female cultured sturgeon hybrid, the bester. Journal of Fish Biology 48: 1164-1178.
- Webb MAH, Feist GW, Foster EP, Scherck CB, Fitzpatrick MS (2002) Potential classification of sex and stage of gonadal maturity of wild white sturgeon using blood plasma indicators. Journal of Transactions of the American Fisheries Society 131: 132-142.
- Le-Menn F, Pelissero C (1991) Histological and ultrastructural studies of oogenesis of the Siberian sturgeon. P Williot (Ed) Acipenser, CEMAGRAEF Published: 113-117.
- Amiri BM, Maebayashi M, Adachi S, Yamauchi K (1996) Testicular development and serum sex steroid profiles during the annual sexual cycle of the male sturgeon hybrid, the bester. Journal of Fish Biology 48: 1039- 1050.
- Zhang LZ, Zhuang P, Zhang T (2002) Gonadal development of cultured Amur sturgeon, Acipenser shrenckii. Journal of Fish Sci Chi 9: 321-327.
- Doroshov SI, Moberg GP, Van Eenennaam LP (1997) Observation on the reproductive cycle of cultured white sturgeon, Acipenser transmontanus. Journal of Environmental Biology of Fishes 48: 265-278.
- Silverstein JT, Small BC (2004) Biology and culture of Channel Catfish. In: Tucker CS, Hargreaves JA (Eds). Elsever BV: 69-94.
- Kiabi BH, Abdoli A, Naderi N (1999) Status of fish fauna in the south Caspian basin of Iran. Zoology Middle East 18: 57-65.
- Hochleithner M, Gessner J (2001) The Sturgeon and Paddlefishes of the world. Journal of Biology and Aquaculture: 106.
- Hurvitz A, Jackson K, Degani G, Levavi-Sivan B (2007) Use of endoscopy for gender and ovarian stage determinations in Russian sturgeon, Acipenser gueldenstaedtii, grown in aquaculture. Journal of Aquaculture 270: 158- 166.
- Roff DA (1983) An allocation method of growth and reproduction in fish. Canadian Journal of Aquatic science 40: 1395-1404.
- Lowe-Mc Connell RH (1982) Tilapias in fish communities. In: Pullin RVS, Lowe-McConnell RH (Eds) Proceedings of the International Conference on the Biology and Culture of Tilapias 1: 83-113.
- Liu SJ (1991) Studies on the origin and migration of the primordinal germ cells and gonad differentiation in Clarias lazera. Acta Hydrobiologica Sinica 15: 1-7.
- Srijunngam J, Wattanasirmkit K (2001) Histological structures of Nile Tilapia, Oreochromis niloticus, ovary. The Natural History Journal of Chulalongkorn University 1: 53-59.
- Abou-Seedo F, Dadzin S, Al-Anaan KA (2003) Histology of ovarian development and maturity stages in the yellowfin seabream, Acanthopagrus latus, reared in cages. Kuwait Journal of Science and Engineering 30: 121-137.
- Urbatzka R, Rocha MJ, Rocha E (2011) Hormones and reproduction of vertebrates. In: Norris DO, Lopez KH (Eds). Regulation of ovarian development and function in teleost, Elsever, UK: 84-101.
- Dadzie S (1974) Oogenesis and the stage of maturation in the female cichlid fish, Tilapia mossambica. Ghana J Sci 14: 23-31.
- Wiegand MD (1982) Vitellogenesis in fishes. In: Richter CCJ, Goos, JJTh, (Eds). Proceeding of international symposium on reproductive physiology in fish.
- Dadzie S, Abou-Seedo F, Al-Shallal T (2000) Histological and gistochemical study of oocyte development in the silver pomfret, Pampus argenteus in Kuwait water. Arabian Gulf Journal of Scientific Research 18: 23-31.
- Alavi SM, Cosson J, Karami M, Amiri BM, Akhoundzadeh MA (2004) Spermatozo amotility in the Persian sturgeon, Acipenser persicus: effects of pH, dilutionrate, ions and osmolality. Reproduction 128: 819-828.
|
| Â |
| Â |