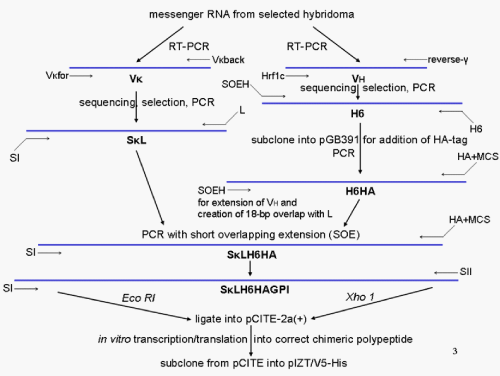
Figure
6: Flow chart for the cloning strategy of the pIZT/V5-His vector harbouring the SK LH6F fusion cassette that encodes the open reading frame for the GPI-modified
chimeric anti-protein analyte scFv harbouring the elements as described in Figure 5. Total RNA was extracted from freshly prepared hybridoma cells, precipitated, dissolved and then used for first strand cDNA synthesis in the presence of an oligo(dT)18 primer. The variable (V) regions of the К light chain and heavy (H) chain genes expressed by the selected hybridoma cells were amplified from the template cDNA using Vκfor and Vκback primers for the light chain domain or Hrf1c forward plus reverse γ-primers for the heavy chain domain [167]. The primers for cloning of the VH and VK domains and generation of the 270-codon SκL-H6HA fusion cassette, a hexa-histidine-tagged (H6) and influenza hemagglutinin-epitope-tagged (HA) scFv directed against the protein analyte are indicated. The identified, confirmed and sequenced positive clones matched to a high degree to mouse immunoglobulin К or H sequences in BLAST searches of the non-redundant GenBank database. The cassette equipped with the sequences of the secretory signal peptide I hybrid from rat growth hormone and somatotropin (SI) followed by the sequences of the V domain from the К chain and then of the linker peptide (L) was generated by PCR with the corresponding primers. L was formed by two Gly4Ser segments encoded by a 4-to-1 mixture of Gly and Ser codons [168]. Primer H6 was used to introduce the six-histidine purification tag to the carboxy-terminus of the H domain. Primer SOEH was used to drive an 18-bp overlap to trigger the combination of the SκL cassette and the His6 tag cassette for the H domain into an in-frame fusion construct. The full-length overlap product (7335 bp) was cloned into plasmid pGB391 in frame with the HA epitope tag at the carboxy-terminus of the 270-codon chimeric open reading
frame. From this pGB391 variant a PCR product was prepared by amplification with the primers SI and HA+MCS, in which the latter one is located in the multiple cloning site (MCS) of pGB391. Finally, the amplification product SκLH6HA was digested with EcoR1 and Xho1 and then ligated into the pCITE-2a(+) vector and then cloned into TOP10F’ vector in E. coli. This vector harbours a T7 promoter and a 5’-untranslated cap-independent translation enhancer, CITE, that was engineered to support the efficient in vitro transcription/translation in reticulocyte lysates. This was performed in order to confirm the correctness of the putative SκLH6HA open reading frame in this pCITE vector and its ability to drive the synthesis of a fusion protein of the expected size.
For the introduction of the carboxy-terminal signal sequence II directing the GPI modification of the SκLH6HA fusion protein, PCR primers were derived from the cDNA sequence of human placental alkaline phosphatase (PLAP; GenBank file M13077). The resulting PCR product of a first amplification reaction was elongated in a second PCR reaction using the primers, NH3 and NH4, which ensure overlap with the HA tag (NH3) and harbour a Xba1 restriction site (NH4). This PCR product was combined at equimolar stoichiometry with the SκLH6HA amplicon and then used for another PCR reaction with the primer NH5 (harbouring an EcoRI restriction site). The resulting two amplicons have an 18-bp overlap within the HA-tag. The final PCR product was digested with EcoR1 and Xba1 and then ligated as a cassette into the plasmid pIZT/V5-His which drives the high-level expression of the scFvLH6HAGPI fusion protein in High FiveTM insect cells. As a control, a second chimeric protein, scFvH6HA, lacking the GPI anchor was generated upon omission of the two final PCR amplifications. |