 |
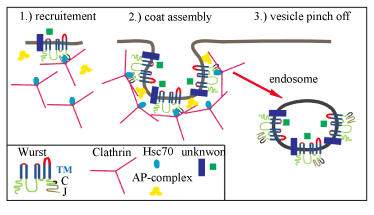
| Figure 5: The evolutionarily conserved DnaJ-domain protein Wurst is involved in endocytosis (A) The cartoon illustrates the putative role of Wurst in endocytosis of clathrincoated vesicles. The predicted Wurst structure contains six transmembrane domains (TM; blue), a clathrin-binding motif (C; black) and a J-domain (J; grey).Wurst cytoplasmic parts are indicated in green, the extracellular in red. Clathrin assembles a coat-like structure around the nascent vesicle, aided by adaptor proteins (AP-complex). After vesicles scission, clathrin disassembles and can be reused for a new round of endocytosis. The uncoated vesicle pinches off and fuses with acceptor compartments such as early endosomes. Our data indicate that Wurst recruits clathrin and Hsc70-4 to the apical membrane. In contrast to clathrin and Hsc70-4, Wurst remains in the vesicle, which fuses to acceptor compartments, such as early endosomes. Thereby, Wurst may coordinate the endocytosis of unknown factors, which are involved in airway liquid-clearance. |