 |
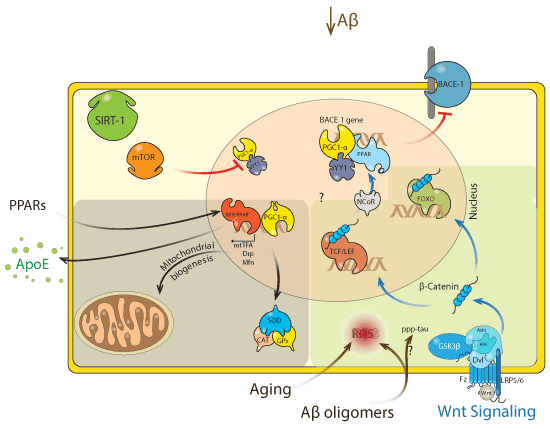
| Figure 5: Cross-Talk among SIRT1, PPARs, PGC1α and Wnt signaling. The figure summarizes the current knowledge regarding key interactions between SIRT1/ PPARs/PGC1α axis and other major cellular signaling, like the Wnt pathway. Both aging and Aβ play a critical role in AD pathogenesis, leads to cellular oxidant/ antioxidant unbalance because increased ROS production affects normal cell physiology. SIRT1 has a remarkable activity against aging and neurodegeneration, particularly against the damage induced by Aβ. According to our knowledge, SIRT1 should act following at least two close related mechanisms, in the first one SIRT1 exerts an inhibitory action over mTOR, an event that induces the release of the PGC1α from the YY1/PGC1α complex allowing the recruitment of the PGC1α factor as a transcriptional co-activator of the RXR:PPAR heterodimer. On the other hand, SIRT1 is able to down-regulate the expression of BACE1, a critical amyloidogenic enzyme, by a mechanism which involves the binding of the PPAR:PGC1α complex to the PPRE in the promoter region of the BACE1 gene; allowing the recruitment of transcriptional co-repressors, such as YY1 and NCoR, limiting the expression of BACE1, and leading to the reduction of the Aβ levels. These effects evidence the complementarity and perhaps the simultaneity of the SIRT1 pathways. Furthermore, considering the outcome of the RXR:PPAR transcriptional activity, which includes increased antioxidant activity (SOD/CAT/GPx), mitochondrial protection (DRP1/Mfn/mtTFA), and increased ApoE expression, a key Aβ-clearance protein, the complementarity of the SIRT1 pathways seems more than probably. Additionally, it has been proposed that ROS are able to disrupt the Wnt singling pathway, directing β-catenin, the effector molecule of the Wnt pathway, from Tcf/Lef to FOXO, arresting cell proliferation (Wnt) and promoting cell senescence (FOXO). At this point, the increased antioxidant activity and mitochondrial health induced by SIRT1 activity should lead to reduced ROS levels, preventing the disruption of the Wnt signaling pathway. In the same way, NCoR has been also described as a Wnt target-gene transcriptional co-repressor, being possible to hypothesize that the YY1/NCoR SIRT1- mediated recruitment might induces an increased in Wnt target-genes expression because of the redirection of the NCoR. |