 |
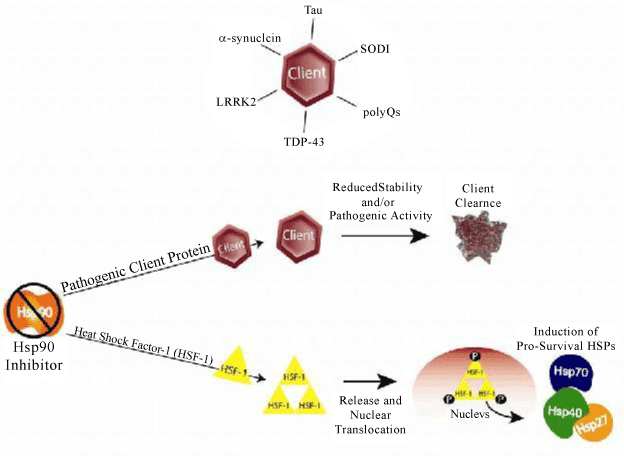
| Figure 1: Inhibition of Hsp90 generates two distinct mechanisms both of which result in client protein degradation and enhanced cell survival. Pathogenic Hsp90 “client” proteins are dependent on Hsp90 for nascent folding and maintenance of structure. Upon Hsp90 inhibition, client proteins, including those involved in disease lose stability and are degraded. Hsp90 inhibition also promotes the induction of other heat shock proteins through an HSF1 dependent mechanism. These HSPs can also promote clearance and block aggregation of aberrant clients while enhancing cell survival under the “stressed” condition. |