 |
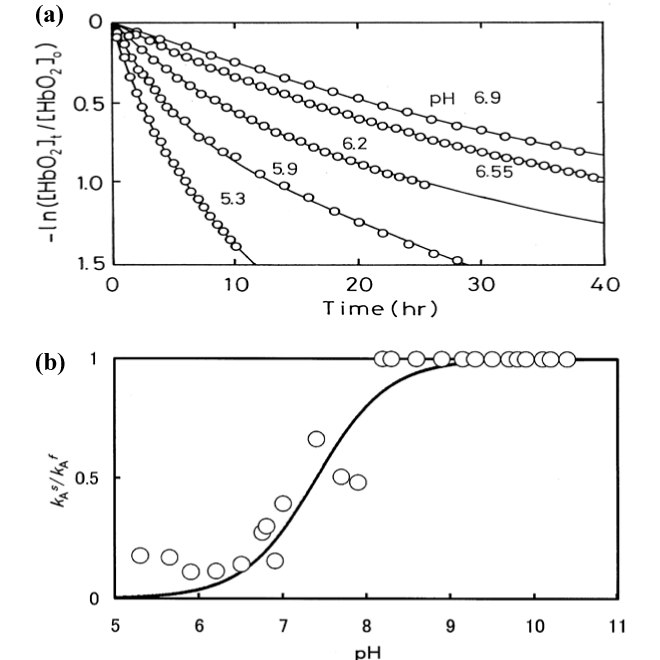
| Figure 4: Oxidative behavior of human Hb (HbO2) in various buffer conditions. (a) A−ln([HbO2]t/[HbO2]0) versus time plot (first−order plot) for human HbO2 autoxidation. Two−phase autoxidation with an initial fast reaction (kAf) followed by a second slower reaction (kAs) seen in acidic−to−neutral pH ranges. Solid lines represent the leastsquares fitting to experimental data in each run. Conditions: HbO2 concentration was 50−125 μM (in heme contents); and the reaction proceeded in 0.1 M buffer at 37°C. (b) A kAskAf versus pH plot for human HbO2 autoxidation. The solid line stands for the best-suited fitting under the assumption that a single, dissociable group, AH with Ka, can be involved in the reaction. This was achieved with the setting pKa= 7.4. |