 |
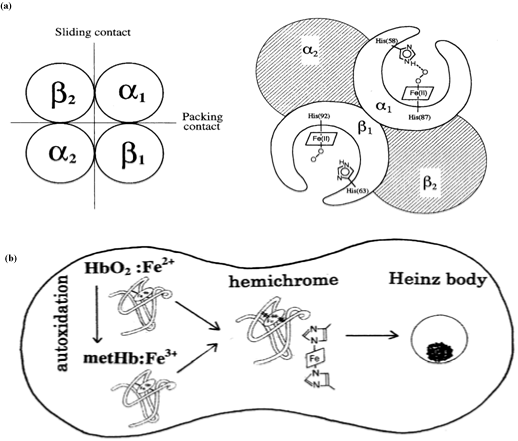
| Figure 13: Schematic representation of the role of the α1−β1 (and α2−β2) interface in human HbO2.(a) The leftfigure shows a molecular dyad axis of Hb tetramer (which is perpendicular to the plane of the figure) relating the α1β1 dimer to the α2β2 dimer and consisting of the two different types of αβ contacts: one is the α1−β1 (and α2−β2) (packing contact); and the other is the α1−β2 (and α2−β1) (sliding contact). The right figure illustrates that the α1−β1 (and α2−β2) produces in the β chain a tilting of the distal (E7) His residue, thereby preventing the proton-catalyzed displacement of O2− by a solvent water molecule. (b) The figure demonstrates that depending on internal and extraneous circumstances of the erythrocyte including pH and temperature, the α1−β1 (and α2−β2) interface produces a conformational constraint in the constituted chains via tilting of the distal His (E7) residues so as to cause degradation of the Hb molecule to hemichrome, and subsequent Heinz-body clustering within the erythrocyte. In the spleen, Heinz body-containing red cells become trapped and hence undergo hemolysis. |