
Page 39
conferenceseries
.com
Volume 7, Issue 2 (Suppl)
J Phys Chem Biophys, an open access journal
ISSN: 2161-0398
Electrochemistry 2017
July 10-11, 2017
Electrochemistry
3
rd
International Conference on
July 10-11, 2017 Berlin, Germany
Iodides confinement within activated carbon porosity resulting battery-type electrode for aqueous hybrid
supercapacitor
Qamar Abbas, Patryk Przygocki, Paulina Babuchowska
and
Francois Beguin
Poznan University of Technology, Poland
S
upercapacitors (SCs) generally use activated carbon (AC) electrodes and organic electrolytes e.g. 1 mol L
-1
TEABF4 in acetonitrile
due to the energy vs. voltage square dependence. However, due to the flammable character of acetonitrile, environment-friendly
and low cost alternatives e.g. neutral aqueous Li
2
SO
4
(pH=6.5-7.0) exhibiting moderate voltages up to 1.5 V in SCs have been recently
proposed. Such voltage exceeding water stability of 1.23 V is due to large over-potential for di-hydrogen evolution at the negative
carbon electrode caused by local downshift of pH. Lately, by introducing potassium iodide (KI) in aqueous Li
2
SO
4
, AC/AC hybrid
cells operating up to 1.6 V displayed high capacitance as a result of hybridization of a battery-type positive electrode and capacitor-
type negative one. The battery-type performance of the positive electrode is associated with redox reactions 2I-
↔
I
2
+ 2e- enhancing
greatly the capacity of the positive electrode than for the negative one, C
+
>>C-, and using equation for capacitors in series 1/C=1/
C
+
+1/C- capacitance of cell is equal to negative electrode capacitance. Here, we show that hybrid capacitors in aqueous KI+Li
2
SO
4
(pH=6.5) using symmetric carbon configuration losses capacitance upon cycling/floating at 1.5 V. When using microporous carbons
as positive and negative electrodes, the former reaches to +0.692 V vs. SHE, and when implementing mesoporous electrodes, the
negative electrode reaches to -0.985 V vs. SHE well below the di-hydrogen evolution potential (-0.46 V vs. SHE). Hence, both systems
display capacitance loss under cycling/floating at 1.5 V. We implement asymmetric configuration using mesoporous carbon as
positive electrode to better trap iodide species, and microporous carbon as negative one to improve hydrogen storage, to balance the
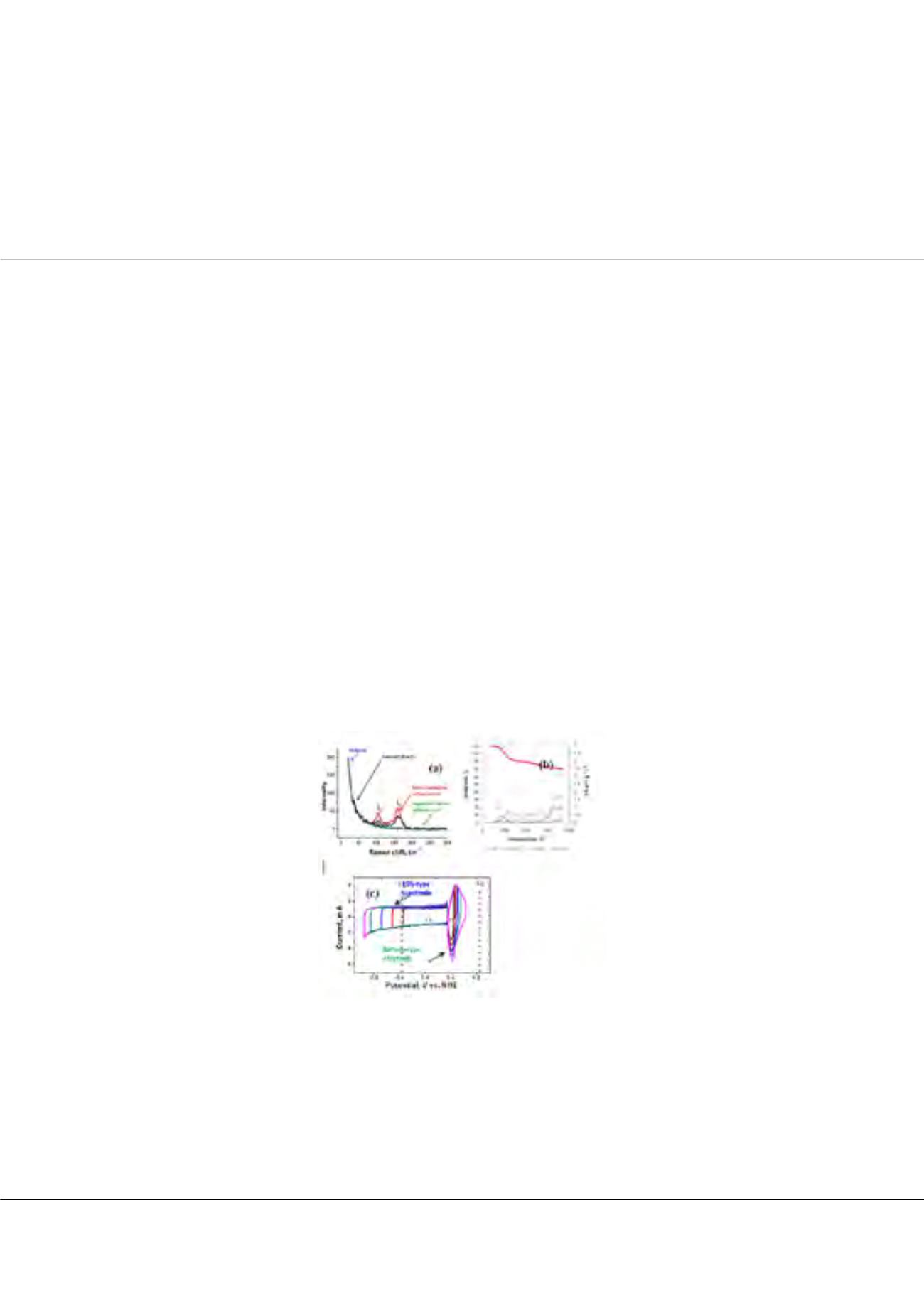
system. TPD, Raman, gas adsorption and electrochemical data on electrodes and cells (Figure 1) proves that the oxidation of positive
electrode and hydrogen production on negative electrode are reduced, improving the cyclability, capacitance and energy efficiency of
the cell up to 10,000 cycles.
Biography
Qamar Abbas received his PhD degree in Technical Sciences from Graz University of Technology (Austria) in 2011. From 2011-2015, he did his Post-doctoral
studies from the Institute of Chemistry and Technical Electrochemistry (ICTE) at Poznan University of Technology, Poznan, Poland. Since 2016, he is an Assistant
Researcher at the ICTE and his research interests are related with optimization of supercapacitor performance in aqueous and organic electrolytes under testing
conditions and corrosion investigations and mitigation of stainless steel type alloys in aqueous media.
qamar.abbas@put.poznan.plQamar Abbas et al., J Phys Chem Biophys 2017, 7:2(Suppl)
DOI: 10.4172/2161-0398-C1-019
















