
Page 50
conferenceseries
.com
Volume 10
Journal of Bioanalysis & Biomedicine
ISSN: 1948-593X
Euro Biosimilars 2018
April 26-27, 2018
April 26-27, 2018 Rome, Italy
11
th
EUROPEAN BIOSIMILARS CONGRESS
Automated permethylation for glycosylation analysis of biologics using MALDI-TOF-MS
Archana Shubhakar
1,3,
Daniel Spencer
1
, Daryl Fernandes
1
and
Manfred Wuhrer
2
1
Ludger Ltd, UK
2
Leiden University Medical Center, The Netherlands
3
VU University Ámsterdam, The Netherlands
F
or most therapeutic glycoproteins the glycosylation patterns correlate strongly with the clinical safety and efficacy profiles.
In biological tissues these patterns can also correlate with the state of health or disease of the individual. Given this, there is
increasing interest in accurately characterizing changes in glycosylation, for example in Quality by Design studies throughout
biopharmaceutical development as well as in glycan biomarker discovery for medical diagnostics. Changes in glycosylation
patterns can be complex and subtle and the numbers of samples needed to be analysed can be large, ranging from hundreds
to thousands. To perform these studies, reliable systems for high-throughput (HT) glycomics are needed. However, despite
many advances in glycosylation analysis there are still problems with current technologies, including low sample throughput,
long turnaround times, high cost per sample and labour intensiveness. This talk concerns “LongBow” — a system developed
at Ludger for reliable HT glycomics. The “LongBow” system is made up of flexible, modular technologies for semi-automated
processing of glycans from a variety of clinical and bio-therapeutic samples and analysis by mass spectrometry (MS) and/or
ultrahigh performance liquid chromatography (UHPLC).The focus here will be on permethylatedN- andO-glycans analysed by
matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). This automated, HT glycan
preparation and permethylation method showed to be robust, convenient and fast and can be applied for biopharmaceutical
glycan profiling and clinical glycan biomarker studies.
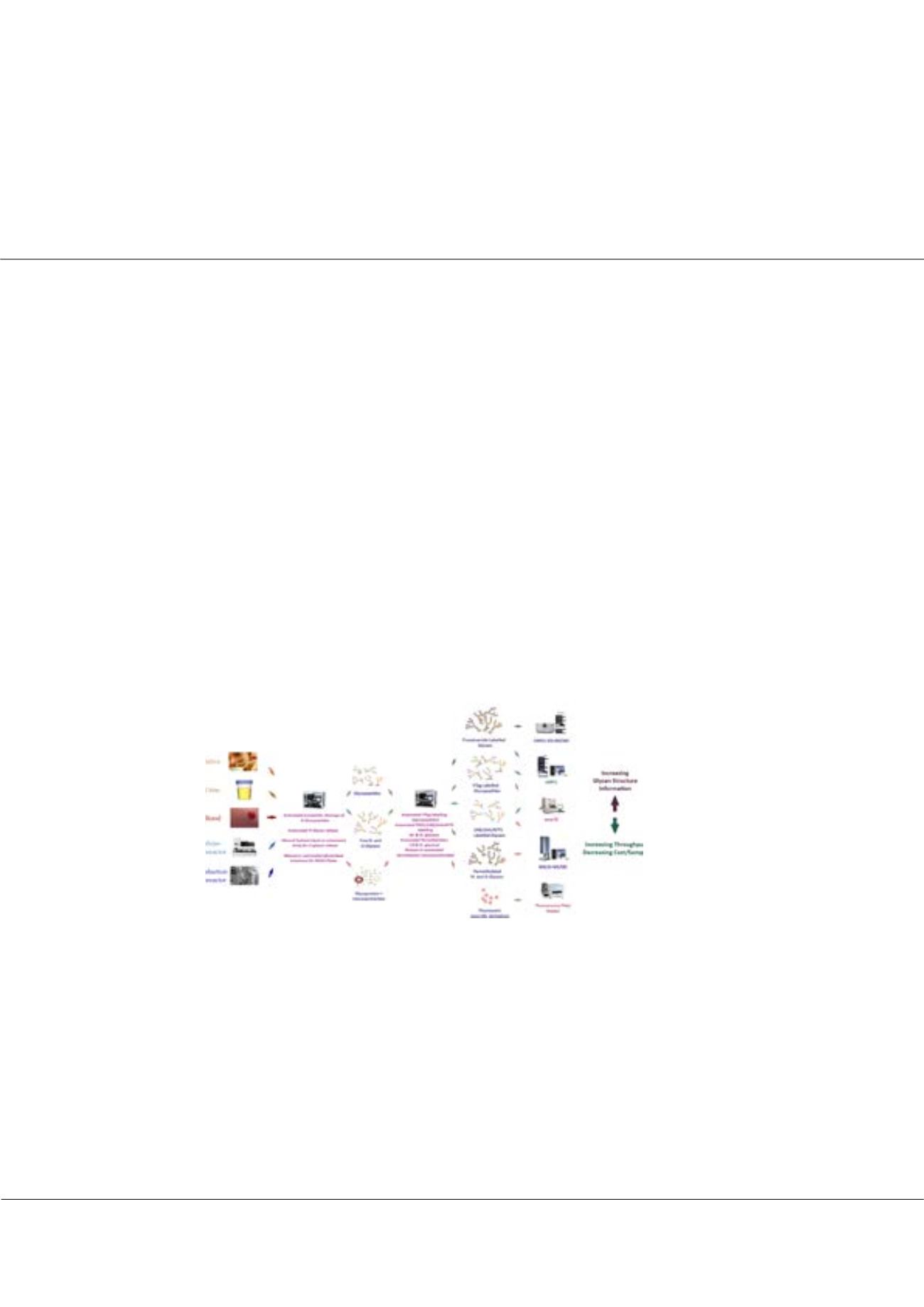
Figure:
“LongBow” system developed at Ludger, is made up of flexible, modular technologies for semi-automated processing
of glycans from a variety of clinical and biotherapeutic samples.
Recent Publications
1. Ventham N T et al. (2016) Integrative epigenome-wide analysis demonstrates that DNA methylation may mediate
genetic risk in inflammatory bowel disease. Nature Communications. 7:13507.
2. Dotz V et al. (2015) Mass spectrometry for glycosylation analysis of biopharmaceuticals. TrAC Trends in Analytical
Chemistry. 73:1-9.
Archana Shubhakar et al., J Bioanal Biomed 2018, Volume 10
DOI: 10.4172/1948-593X-C1-038
















