
Page 34
Notes:
conferenceseries
.com
Volume 8, Issue 2 (Suppl)
Chem Sci J 2017
ISSN: 2150-3494 CSJ, an open access journal
Euro Chemistry 2017
May 11-13, 2017
May 11-13, 2017 Barcelona, Spain
4
th
European Chemistry Congress
The synthesis of complex marine depsipeptides
Judit Tulla-Puche
1
and
Ramon Y Cajal
2
1,2
University of Barcelona, Spain
M
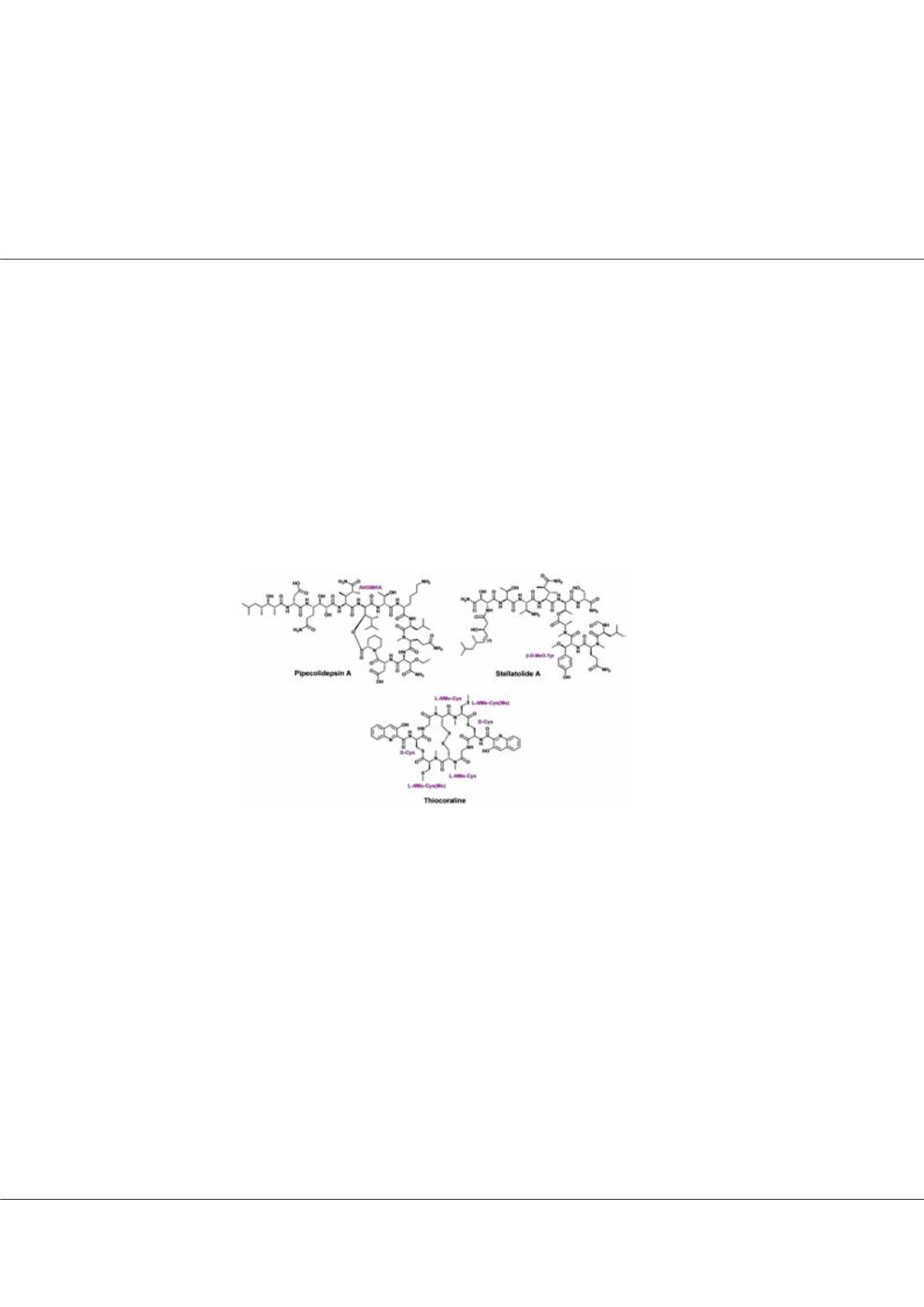
arine organisms are a rich source of bioactive molecules. Among them, cyclodepsipeptides show relevant biological profiles,
mostly including cytotoxic and anti HIV activities, and they are, therefore, promising candidates for medicinal chemistry
programs. Herein, we report the synthesis of the potent antineoplastic agents pipecolidepsin A and Stellatolide A, “head-to-side-
chain” cyclodepsipeptides, where the C-terminus is linked to a -hydroxy group via an ester bond, and of thiocoraline, a byciclic
thiodepsipeptide that acts as bisintercalator. The three molecules present extremely challenging structures. Pipecolidepsin A bears the
unprecedented and extraordinary bulky AHDMHA residue at the branching point, which makes the construction of the extremely
hindered ester bond the major synthetic challenge to overcome. On the other hand, the high propensity of the unnatural -MeO-
D-Tyr residue in Stellatolide A to suffer decomposition is the main limitation of its assembly. Finally, Thiocoraline’s large amount of
cysteines in a rather small structure represents the principal restraint. The successful solid-phase synthetic strategies that resulted in
the three synthetic and active cyclodepsipeptides will be discussed.
Biography
Judit Tulla Puche received her Ph.D. in organic chemistry (2004) from the University of Minnesota under the supervision of Prof. George Barany. Her thesis dealt
with the solid-phase synthesis of small proteins. After finishing her doctoral studies, she joined the group of Prof. Fernando Albericio at the Institute for Research in
Biomedicine (IRB) where she became Research Associate, working on the synthesis of marine antitumor depsipeptides and complex peptides. In 2015, and after
obtaining a Ramon y Cajal contract, she moved to the Department of Organic Chemistry at the University of Barcelona to establish her own research group. Her
research interests span a broad range of topics at the interfaces of peptide chemistry and chemical biology.
judit.tulla@ub.eduJudit Tulla-Puche et al., Chem Sci J 2017, 8:2(Suppl)
http://dx.doi.org/10.4172/2150-3494-C1-008















