| Research Article |
Open Access |
|
| Shweta U. Tavasalkar1*, H. N. Mishra2 and Sanjith Madhavan3 |
| 1Food Engg. Deapartment, IIT Kharagpur, Maharashtra, India |
| 2Professor, Agriculture and Food Engg. Deapartment, IIT Kharagpur, Kharagpur, West Bengal, India |
| 3Synthite Industries Ltd, Kolenchery, Cochin, Kerala, India |
| *Corresponding authors: |
Shweta U. Tavasalkar
Food Engineering Deapartment
IIT Kharagpur, Maharashtra, India
Tel: +91 9673105052
E-mail: shwetz24@gmail.com |
|
| Â |
| Received October 11, 2012; Published December 06, 2012 |
| Â |
| Citation: Tavasalkar SU, Mishra HN, Madhavan S (2012) Evaluation of Antioxidant Efficacy of Natural Plant Extracts against Synthetic Antioxidants in Sunflower Oil. 1:504. doi:10.4172/scientificreports.504 |
| Â |
| Copyright: © 2012 Tavasalkar SU, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| With the improvement in living conditions, consumers increasingly reject food prepared with additives of chemical origin due to health issues. Four natural antioxidant blends prepared with hexane and acetone extract of rosemary, green tea and other additives having synergistic effect were chosen viz. OF001, OF002, OF003 and OF004 for the present study. HPLC analysis of these extracts showed that OF001 contained 4.789% carnosic acid. Similarly, OF002, OF003 and OF004 contained 3.477%, 4.676% and 7.074% carnosic acid respectively. Total phenolic content of OF001, OF002, OF003 and OF004 were found as 89.64, 89.64, 81.36, 28.29 and 77.68 mg GAE/g, respectively. Control sunflower oil found more susceptible to oxidative deterioration as peroxide value of control sunflower found to increase rapidly after 15 days of storage at ambient temperature. For sunflower oil, OF002@ 0.1% was found effective to restrict the peroxide value below threshold level up to 120 days of storage. |
| Â |
| Keywords |
| Â |
| Natural antioxidant; Rosemary; Sunflower oil; Rancimat |
| Â |
| Abbrevations |
| Â |
| AV: P-Anisidine Value; BHA: Butylated Hydroxy Anisole; BHT: Butylated Hydroxy Toluene; FCR: Folin–Ciocalteu Reagent; FFA: Free Fatty Acids; GAE: Gallic Acid Equivalents; OSI: Oil Stability Index; PG: Propyl Gallate; PUFA: Polyunsaturated Fatty Acid; PV: Peroxide Value; SFO: Sunflower Oil; TBHQ: Tertiary Butyl Hydroquinone; TPC: Total Polar Compound |
| Â |
| Introduction |
| Â |
| The oils and fats of commerce are mixtures of lipids. They are mainly triacylglycerols (generally >95%) accompanied by diacylglycerols, mono-acylglycerols and free fatty acids, but they may also contain phospholipids, free sterols and sterol esters, tocols (tocopherols and tocotrienols), triterpene alcohols, hydrocarbons and fat-soluble vitamins. During refining, some of the minor components are removed, wholly or in part, and useful materials may be recovered [1]. Sunflower oil is one of the most popular vegetable oils and in some countries it is preferred to soybean, cottonseed and palm oils. The sunflower is the fourth largest oil source in the world, after soybean, palm and canola oil. Demand for sunflower oil increased sharply in the mid-eighties when high polyunsaturated fatty acid (PUFA) margarine became the desired table margarine for health reasons. Traditional sunflower oil is excellent for cooking, making salad dressing, margarine, and so on, but it cannot be used for manufacturing shelf-stable fried foods because of its poor oxidative stability [1]. Fats, oils and lipid-based foods deteriorate through several degradation reactions both on heating and on long term storage. The main deterioration processes are oxidation reactions and the decomposition of oxidation products which result in decreased nutritional value and sensory quality. The retardation of these oxidation processes is important for the food producer and, indeed, for all persons involved in the entire food chain from the factory to the consumer. Oxidation may be inhibited by various methods including prevention of oxygen access, use of lower temperature, inactivation of enzymes catalyzing oxidation, reduction of oxygen pressure, addition of chelating agent and the use of suitable packaging [2]. Another method of protection against oxidation is to use specific additives which inhibit oxidation. These are correctly called oxidation inhibitors, but nowadays are mostly called antioxidants. According to Halliwell and Gutteridge, the term antioxidant refers to ‘‘a substance significantly delays or prevents oxidation of that substrate’’. Antioxidants delay the development of off-flavors by extending the induction period. Addition of antioxidants after the end of this period tends to be ineffective in retarding rancidity development [2]. There has been an increasing interest in the use of natural antioxidants, such as tocopherols, flavonoids and rosemary (Rosmarinus officinalis L.) extracts for the preservation of food materials in recent years [3-6], because these natural antioxidants avoid the toxicity problems which may arise from the use of synthetic antioxidants, such as butylated hydroxy anisole (BHA), butylated hydroxy toluene (BHT) and propyl gallate (PG) [7,8]. Reports revealing that BHA and BHT could be toxic, and the higher manufacturing costs and lower efficiency of natural antioxidants such as tocopherols, together with the increasing consciousness of consumers with regard to food additive safety, has created a need for identifying alternative natural and probably safer sources of food antioxidants [9,10]. Natural antioxidants are more ideal as food additives, not only for their free radical scavenging properties, but also on the belief that natural products are healthier and safer than synthetic ones; thus, they are more readily acceptable to the modern consumers [11]. There is at present increasing interest both in the industry and in scientific community for spices and aromatic herbs because of their strong antioxidant and antimicrobial properties, which exceed many currently used natural and synthetic antioxidants. These properties are due to many substances, including some vitamins, flavonoids, terpenoids, carotenoids, phytoestrogens, minerals, etc. which render spices and some herbs or their antioxidant components for preservative properties in food. There has been increasing research in the role of herbs and spices as natural preservatives. Herbs and spices are not just valuable in adding flavor to foods, but their antioxidant activity also helps to preserve foods from oxidative deterioration thereby increasing their shelf life. As an example, ground black pepper has been found to reduce the lipid oxidation of cooked pork [12]. The present was therefore, undertaken in line of the above points, to study the effect of natural antioxidant blends on the oxidative stability of sunflower oil. |
| Â |
| Materials and Methods |
| Â |
| Materials |
| Â |
| Sunflower oil treated with natural antioxidant blends viz. OF001, OF002, OF003 and OF004, two synthetic antioxidants viz. tertiary butyl hydroquinone (TBHQ) and butylated hydroxyl anisole (BHA) and control sample i.e., without any antioxidant were stored at ambient temperature for 180 days and at 50°C for 90 days. Tests at 50°C were carried out in standard laboratory oven. Samples were withdrawn at 15 days interval and tested for quality parameters viz. peroxide value, free fatty acids, p-anisidine value and polar compounds. All experiments were carried out in triplicates. |
| Â |
| Preparation and analysis of natural antioxidant blends |
| Â |
| The natural antioxidant blends were prepared by using rosemary, green tea and other additives with different concentrations. Rosemary Ooty variety (aqueous acetone extract) and rosemary Moroccan variety (pure hexane extract) and spray dried green tea were used for preparing final testing samples viz. OF001, OF002, OF003 and OF004. Lecithin and citric acid are used for synergistic effect. Carnosic acid and carnosol content determination was carried out by using method followed by [13]. In this method, HPLC column, Prodigy ODS-2 150 x 4.6 mm, 5 micron was used as a stationary phase and mixture of acetonitrile (0.1%) and phosphoric acid (60:40) was used as a mobile phase at the rate of 1 ml/min. The detection was carried out at 230 nm. The standards of known concentration of carnosic acid (49.69%) and carnosol (2.42%) were used for preparing standard curve. |
| Â |
| Total phenolic content |
| Â |
| The total phenol content of plant extracts was determined using Folin–Ciocalteu reagent (FCR) method according to the procedure reported by [14] with some modifications. For measuring total phenolic content, gallic acid standard solution in different concentrations (5-80 mg / l) were prepared and standard curve of total phenolic content in terms of gallic acid equivalents (GAE) was prepared. 0.05g - 0.1g of sample was weighed and dissolved in alcohol and volume was made up to 25 ml. From this sample solution, 1.8 ml of solution was transferred in another volumetric flask to which 3.6 ml of 10% aqueous FCR and 14.6 ml of 100 mM sodium carbonate solution was added. The samples were kept for 2 hrs at room temperature for incubation. After 2 hrs, absorbance was measured against reagent blank (alcohol) at 765 nm using 2010 UV-IS Spectrophotometer. |
| Â |
| Oxidative stability of sunflower oil |
| Â |
| The American Oil Chemists Society standard methods (Official Methods and Recommended Practices, 1994) were used for free fatty acids (Ca 5a-40), peroxide value (Cd 8- 53), and p-anisidine value (Cd 18-90). Polar compounds present in oil and fats were measured by IUPAC standard method 2.507. The oil stability index was determined using the Metrohm rancimat (Metrohm 743). |
| Â |
| Statistical analysis |
| Â |
| All experiments were conducted in triplicates and results were represented as mean ± standard deviation. Curve fitting was done and regression coefficient of the equation for best fitted curve was calculated by using Microsoft Excel 10. |
| Â |
| Result and Discussion |
| Â |
| Analysis of natural antioxidant blends |
| Â |
| Hexane and aqueous acetone extract of rosemary and other additives having synergistic effect were used to make blends of natural antioxidant. Four natural antioxidant blends were chosen viz. OF001, OF002, OF003 and OF004 for the present study based on induction time given by accelerated study using rancimat. Carnosic acid (%), carnosol (%) and total phenolic content (mg GAE/g) of OF001, OF002, OF003 and OF004 has been listed in table 1. |
| Â |
|
|
Table 1: Analysis of natural plant extract. |
|
| Â |
| Oil stability index |
| |
| Â |
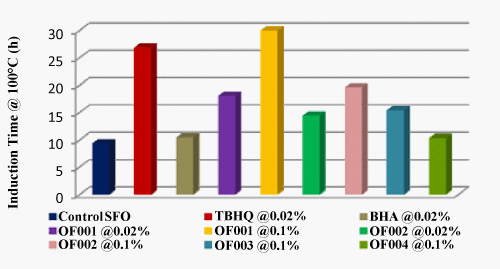
| The oil stability index (OSI) was determined using the Metrohm Rancimat (Metrohm 743). Figure 1 set out the accelerated study of sunflower oil, which is displayed in terms of induction period i.e., the time, elapsed between starting of the oxidation process and occurrence of the secondary reaction products. Table 2 shows the induction time (h) of sunflower oil at 100°C. From table 2, it can be seen that OF001 and OF002 showed oil stability index greater than TBHQ for sunflower oil at 0.1% dosage. Also OF003 and OF004 treatments gave better OSI at 0.1% dosage than BHA. |
| Â |
|
|
Figure 1: Effect of antioxidants on induction time of sunflower oil at 100°C. |
|
| Â |
|
|
Table 2: Accelerated study using Rancimat. |
|
| Â |
| Effect on peroxide value |
| Â |
| Peroxide value (PV) is a measure of the concentration of peroxides and hydroperoxides formed during the initial stages of lipid oxidation. Peroxide value is one of the most widely used tests for oxidative rancidity in oils and fats. For this, oxidation degree on sunflower oil samples was determined by measuring PV in the absence and presence on antioxidants at ambient temperature for 180 days and at 50°C for 90 days. The influence of different antioxidants on PV of sunflower oil samples is shown in Figure 2. From figure 2, it is clear that control sunflower oil has crossed the threshold limit of PV according to CODEX Standards for edible fats and oils (10 meq. of peroxide/kg oil) after 15 days of storage. Also, control sunflower oil has reached the maximum PV (92.9092 ± 0.02464 meq. of peroxide/kg oil) after 180 days of storage at ambient temperature. From figure 3, it can be seen that there is linear increase in PV of control sunflower oil after 15 days. Also, incorporation of BHA and OF004 were not found to be effective in retarding the oxidation of sunflower oil. On the other hand, sunflower oil treated with OF001 and OF002 has shown better results than control sunflower oil even after 120 days of storage in inhibiting the oxidation of sunflower oil at ambient temperature and TBHQ was found to reach threshold limit on day 180. At 50°C, control sample attained its peak value (50.8615 ± 0.104838) on 60th day of storage as shown figure 3. The figure 3 set out the effect of antioxidants on peroxide value of sunflower oil stored at 50°C temperature. Figure 3 has shown that, after day 60, there was decrease in PV. This is due to the commencement of secondary oxidation products which were measured by p-anisidine value. The sinusoidal nature of PV curve was observed for all the treatments for sunflower oil at 50°C. This may occur at elevated temperature as peroxides are tend to form and degrade at higher temperature. |
| Â |
|
|
Figure 2: Effect of antioxidant type and concentration on peroxide value of sunflower oil at ambient temperature. |
|
| Â |
|
|
Figure 3: Effect of antioxidant type and concentration on peroxide value of sunflower oil at 50°C. |
|
| Â |
| Effect on p-anisidine value |
| Â |
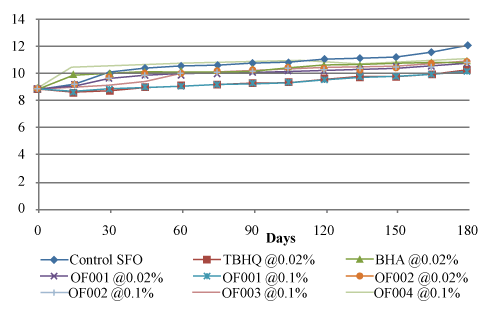
| P-Anisidine value (AV) plays an important role in the oxidation process of edible oil and edible fats. It measures the secondary oxidation products produced during the oxidative degradation of oil, was determined by reacting p-anisidine with the oil in iso-octane and measuring the resultant color at 350 nm. This test has an enhanced sensitivity for unsaturated aldehydes, especially 2,4-dienals, but does not measure the ketonic secondary products of oxidation. The influence of different natural antioxidants treatments on p-anisidine value of sunflower oil during storage at ambient and 50°C temperature are given in figure 4 and figure 5 respectively. Control sunflower oil reached the maximum value (12.072 ± 0.0044) at ambient temperature on day 180. Sunflower oil treated with OF001 @ 0.1% showed minimum increase in AV which reached upto 10.091 ± 0.0042 from initial AV of 8.851 ± 0.0059. Also sunflower oil treated with OF002 @ 0.1% showed lesser increase in AV (10.767 ± 0.0023) as compared to the sunflower oil treated with TBHQ (10.285 ± 0.0025) and BHA (10.93 ± 0.0034). |
| Â |
|
|
Figure 4: Effect of antioxidant type and concentration on p-anisidine value of sunflower oil at ambient temperature. |
|
| Â |
|
|
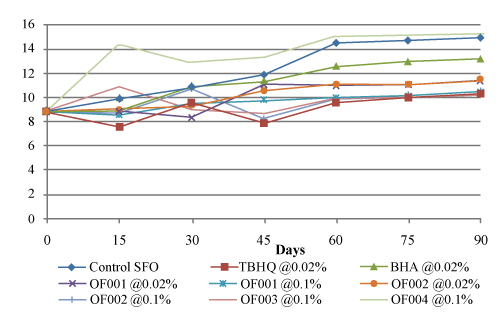
Figure 5: Effect of antioxidant type and concentration on p-anisidine value of sunflower oil at 50°C. |
|
| Â |
| As shown in figure 5, it was seen that at 50°C, control sunflower reached the maximum AV up to 14.897 ± 0.039668 from initial value of 8.851 ± 0.005926. Here also at 50°C, sunflower oil treated with OF001 @ 0.1% showed minimum increase in AV up to 10.312 ± 0.012577. Sunflower oil treated with OF002 @ 0.1% showed lesser increase in AV (10.424 ± 0.010829) as compared to sunflower oil treated with TBHQ (10.305 ± 0.143942) and BHA (13.19 ± 0.011942). |
| Â |
| Effect on free fatty acid |
| Â |
| Free fatty acid (FFA) is an important fat quality indicator during each stage of fats and oils processing. It is a measure of deodorizer efficiency and a process control tool for other processes. In crude fat, FFA or acid value estimates the amount of oil that will be lost during refining steps designed to remove fatty acids. In refined fats, a high acidity level means a poorly refined fat or fat breakdown after storage or use. As shown in figure 6, control sunflower showed the increase in FFA from 0.0503 ± 0.000041% as oleic acid up to 0.0602 ± 0.000006% as oleic acid after 180 days storage at ambient temperature. At 50°C, control reached the maximum value upto 0.0646 ± 0.00005 as oleic acid after 90. From figure 6 and figure 7 it can be seen that sunflower oil treated with OF001 @ 0.02% and OF002 @ 0.02% showed lesser increase in FFA value of sunflower oil as compared to other treatments. |
| Â |
|
|
Figure 6: Effect of antioxidant type and concentration on free fatty acid value of sunflower oil at ambient temperature. |
|
| Â |
|
|
Figure 7: Effect of antioxidant type and concentration on free fatty acid value of sunflower oil at 50°C. |
|
| Â |
| Effect on total polar compounds |
| Â |
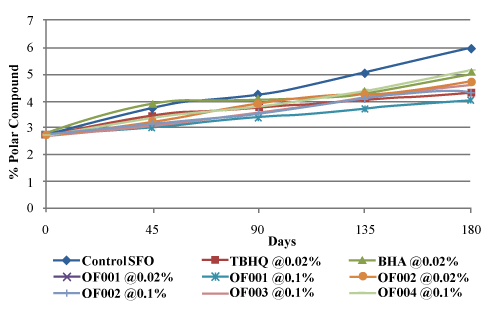
| The purpose of this method is to determine the level of polar, oxidized components in a sample. Faster rate of formation of polar components is cumulative indication of degradation of oil. As shown in figure 8, control sunflower oil reached the maximum value of total polar compounds (TPC) upto 5.931% from initial TPC value of 2.734% after 180 days storage at ambient temperature. Sunflower oil treated with OF001 @ 0.1% showed the minimum value (4.04%) for TPC after 180 days storage at ambient temperature whereas TBHQ gave an increase up to 4.319%. From figure 8 and figure 9, it is seen that addition of antioxidant effectively reduced the formation of polar compounds at ambient temperature and 50°C. Control sunflower oil reached maximum TPC value of 8.1434% after 90 days storage at 50°C. Sunflower oil treated with OF001 @ 0.1% was found to be more effective than TBHQ at ambient temperature whereas at 50°C OF001@ 0.1% reached the TPC value up to 5.4325% against TBHQ (5.1277%). |
| Â |
|
|
Figure 8: Effect of antioxidant type and concentration on total polar compounds of sunflower oil at ambient temperature |
|
| Â |
|
|
Figure 9: Effect of antioxidant type and concentration on total polar compounds of sunflower oil at 50°C. |
|
| Â |
| Conclusion |
| Â |
| During storage of sunflower oil, OF001 and OF002 effectively retarded the primary oxidation up to 120 days while OF003 could prevent the primary oxidation up to 90 days at ambient temperature. At 50°C, all treatments including natural antioxidants and synthetic antioxidant, incorporation of TBHQ were found suitable to retard primary oxidation up to 30 days while oil treated with BHA and control sunflower oil could not prevent primary oxidation even for 15 days. OF001, OF002, OF003 and OF004 were found to inhibit the p-anisidine value of sunflower oil during both storage conditions as effectively as synthetic antioxidants viz. TBHQ and BHA. All the treatments were found to retard secondary oxidation products as compared to control sunflower oil. OF001 and OF002 @ 0.02% showed least increase in FFA value of sunflower oil among all treatments including sunflower oil treated with TBHQ and BHA during both storage conditions. During storage, total polar compound were not found to increase significantly. All the treatments, both natural and synthetic antioxidants were found to keep the value of total polar compound lesser than the control sample. Sunflower oil exhibited more susceptibility to oxidative deterioration as peroxide value of control sunflower was found to increase rapidly after 15 days of storage at ambient temperature. For sunflower oil, OF002@ 0.1% was found effective to restrict the peroxide value within threshold limit up to 120 days of storage. |
| Â |
| Acknowledgements |
| Â |
| Plant extract samples provided by M/s Synthite Industries Ltd., Cochin, Kerala, India for experimental work is thankfully acknowledged. |
| Â |
| |
| References |
| Â |
- Gunstone F D (2004) Vegetable Oils In Food Technology: Composition, Properties and Uses. (2nd ed.). USA.
- Yanishlieva N, Pokorny J, Gordon M (2001) Antioxidants in Food: Practical Applications. (1st edn), Cambridge, USA.
- Bruni R, Muzzoli M, Ballero M, Loi MC, Fantin G, et al. (2004) Tocopherols, fatty acids and sterols in seeds of four Sardinian wild Euphorbia species. Fitoterapia 75: 50-61.
- Frutos MJ, Hernandez-Herrero JA (2005) Effects of rosemary extract (Rosmarinus officinalis) on the stability of bread with an oil, garlic and parsley dressing. Food Science and Technology-LWT 38: 651–655.
- Hras AR, Hadolin M, Knez Z, Bauman D (2000) Comparison of antioxidative and synergistic effects of rosemary extract with a-tocopherol, ascorbyl palmitate and citric acid in sunflower oil. Food Chem 71: 229-233.
- Williams RJ, Spencer JPE, Rice-Evans C (2004) Flavonoids: Antioxidants or signalling molecules? Free Radical Biology and Medicine 36: 838–849.
- Amarowicz R, Naczk M, Shahidi F (2000) Antioxidant activity of various fractions of non-tannin phenolics of canola hulls. J Agri Food Chem 48: 2755–2759.
- Aruoma OI, Halliwell B, Aeschbach R, Loligers J (1992) Antioxidant and pro-oxidant properties of active rosemary constituents: Carnosol and carnosic acid. Xenobiotica 22: 257–268.
- Sherwin ER (1990) Antioxidants. In A. L. Branen, P. M. David- son & S. Salminen, Food antioxidants. New York: Marcel Dekker Inc.
- Wanasundara UN, Shahidi F (1998) Antioxidant and pro-oxidant activity of green tea extracts in marine oils. Food Chem 63: 335-342.
- Chu YH, Hsu HF (1999) Effects of antioxidants on peanut oil stability. Food Chem 66: 29-34.
- Peter KV (2001) Handbook of Herbs and Spices. Vol. 1. (1st ed.). Cambridge, London: Woodhead Publishing Limited, (Chapter 1).
- Okamura N, Fujimoto Y, Kuwabara S, Yagi A (1994) High performance liquid chromatographic determination of carnosic acid and carnosol in Rosmarinus officinalis and Salvia officinalis. J Chromatogr A 679: 381–386.
- Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and 260other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods in Enzymology 299: 152–178.
|
| Â |
| Â |