
Page 43
Notes:
conferenceseries
.com
Joint Conference
July 17-18, 2017 Chicago, USA
International Conference on
DIAMOND AND CARBON MATERIALS & GRAPHENE AND SEMICONDUCTORS
Volume 6, Issue 6 (Suppl)
J Material Sci Eng, an open access journal
ISSN: 2169-0022
Diamond and Carbon 2017 & Graphene 2017
July 17-18, 2017
Fluorination of boron-doped diamond film electrodes for minimization of perchlorate formation
Pralay Gayen
and
Brian P Chaplin
University of Illinois at Chicago, USA
T
he effects of surface fluorination on perchlorate formation rates as well as organic compound oxidation (phenol,
terephthalic acid (TA) rates at boron-doped diamond (BDD) film anodes were investigated. Different fluorination methods
like electrochemical oxidation of perfluorooctanoic acid (PFOA) solutions, radio frequency plasma with H
2
/CF
4
gas, and
silanization with aliphatic (1H, 1H, 2H, 2H perfluorodecyltrichlorosilane) and aromatic triethoxy (pentafluorophenyl) silane)
compounds have been used to incorporate fluorinated functional groups on the BDD surface, which was confirmed by x-ray
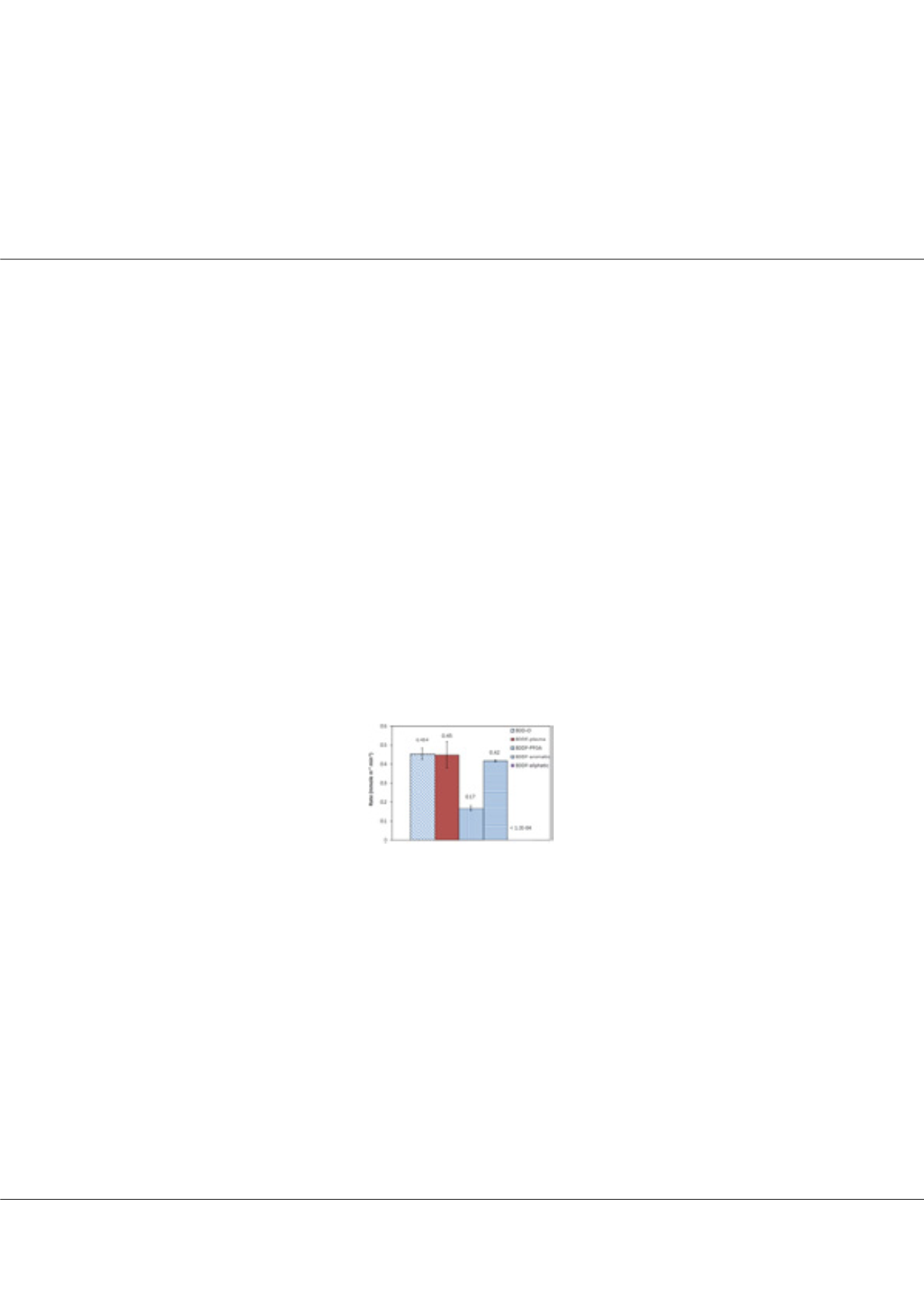
photoelectron spectroscopy. The perchlorate formation rate was lowered by only 63.2% for PFOA modified BDD electrode and
remained similar for both the plasma fluorinated and aromatic silanized BDD electrodes. However the perchlorate formation
rate (<0.12 µmoles m-2min-1) via chlorate and chloride oxidation was lowered by 99.96% and 99.95%, respectively, for aliphatic
silanized electrode. The phenol oxidation rate decreased by 61% and 16.3% (with chloride) and TA oxidation decreased by 55%
for the aliphatic silanized electrode. The apparent selectivity of the aliphatic silanized BDD electrode increased by 2.1-fold and
2.2-fold for TA and phenol, respectively whereas it decreased 680-fold for perchlorate formation. Fe(CN)63-/4- and Fe
3
+/2+
redox couples using cyclic voltammetry indicated that steric hindrance and hydrophobic effects between the fluorinated chains
and chlorate ions may be responsible for the very low perchlorate formation on the aliphatic silanized (<0.12 µmoles m-2 min-
1) and PFOA modified electrodes (0.17 µmoles m-2 min-1). The PFOA modified BDD electrode showed a similar perchlorate
formation rate after ageing, which confirmed high stability of the PFOA modification. The perchlorate formation rate was
below the detection limit (<0.12 µmoles m-2 min-1) for up to 10 consecutive chlorate oxidation experiments and showed
almost 90% decrease in perchlorate formation thereafter, which confirmed very high stability of aliphatic silanization under
OH- production.
Biography
Pralay Gayen has expertise in the area of Electrochemical Treatment of water and wastewater matrix. His research objective is to develop effective, highly
robust and highly selective material to detect antibiotics, degrade different organics and inhibit perchlorate formation in water matrix and these compounds pose
serious health hazard. He has been using boron-doped diamond (BDD) electrodes for wastewater treatment as they are efficient at oxidizing recalcitrant organic
compounds through electrochemical oxidation. He has also been using differently fluorinated BDD electrodes to prevent perchlorate formation without affecting
organic oxidation as perchlorate is carcinogenic and formed through electrochemical oxidation of chloride. He also fabricated a sensor comprising BDD electrodes
modified by carbon nanotube and nafion for the electrochemical detection of antibiotic (ciprofloxacin) in water and wastewater matrix as the presence of antibiotics
for a long time will cause potential emergence of drug resistant bacteria..
pgayen2@uic.eduPralay Gayen et al., J Material Sci Eng 2017, 6:6(Suppl)
DOI: 10.4172/2169-0022-C1-076
















