| Research Article |
Open Access |
|
| Calado C1*, Pereira A.G1, Pinho J.P1, Vieira N.B2, Medeiros R1 and Rodrigues I1 |
| 1Hospital de Faro, EPE. Rua Penedo Leão, 8000-386 Faro, Portugal |
| 2Hospital do Barlavento Algarvio. Sítio do Poço Seco, 8500-338, Portimão |
| *Corresponding author: |
Cláudia Renata Silva Calado
Hospital de Faro, EPE. Rua Penedo Leão
8000-386 Faro, Portugal
Tel: 00351 965 112 797
E-mail: claudiasilvacalado@hotmail.com |
|
| |
| Received May 22, 2012; Published July 26, 2012 |
| |
| Citation: Calado C, Pereira AG, Pinho JP, Vieira NB, Medeiros R, et al. (2012) Head Circumference in Children with Pervasive Development. 1: 111. doi:10.4172/scientificreports.111 |
| |
| Copyright: © 2012 Calado C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Macrocephaly has been considered an early sign in children later diagnosed with pervasive development disorders (PDD). The data nevertheless remain controversial. This study aimed to evaluate head circumference (HC) and HC growth velocity (GV) in the first 36 months in children diagnosed with PDD. |
| |
| We analysed HC in 11 periods in 90 children diagnosed with PDD and 198 controls using a multiple logistic regression model. We found lower HC values in the female PDD population. We found male Asperger disorder (AS) and male autistic disorder (AD) children to have higher HC values than the control male children. Differences between AD and AS were not significant. We found a higher GV in the first six months for all PDD groups compared to control children, although with statistical significance only for AD. |
| |
| We therefore emphasize the importance of HC in the evaluation of children suspected to have PDD, as it corresponds to an early sign and also a cheap, objective and reliable parameter. |
| |
| Keywords |
| |
| Head circumference; Autistic disorder; Asperger syndrome; Pervasive development disorder |
| |
| Abbreviation |
| |
| AS: Asperger Disorder; AU: Autistic Disorder; HCC: Head circumference; PDD: Pervasive Development Disorders; PDD-NOS: Pervasive Development Disorders not otherwise specified; GV: Growth velocity |
| |
| Introduction |
| |
| Pervasive development disorders (PDD) comprise a nosologic group that includes complex neurodevelopmental disorders such as autistic disorder (AD), Asperger disorder (AS) and pervasive development disorder not otherwise specified (PDD-NOS). This group of disorders is characterized by the presence of social deficits, abnormalities in communication and patterns of behavior and interests that are restricted, stereotyped and repetitive [1-3]. Whether these conditions represent a continuum of severity of the same disorder, with autism exhibiting the greatest aberrant neurodevelopment, or whether in fact they consist of distinct disorders with different neurological mechanisms remains unknown [4-6]. |
| |
| Epidemiological investigations have shown an increase in reported rates of PDD, with an estimated prevalence of 1–80/10 000, which corresponds approximately to 0,5-1% of the child population [1-3,7]. |
| |
| The pathological process leading to PDD remains elusive [8,9], although a neurobiological aetiology has been indicated in several investigations [9,10]. Evidence that genes contribute significantly to the development of PDD results mainly from studies of monozygotic and dizygotic twins [4,8,11]. Family studies have demonstrated that first and second degree relatives of affected individuals may display elements of the triad of symptoms without meeting the full criteria for one of the PDD [4]. There is also strong evidence that environmental factors play a role, mainly prolonged or acute oxygen deprivation during foetal life or in delivery, and perinatal exposure to viral infections, drugs and organophosphates [4]. |
| |
| Typical symptoms are often not recognized until the second or third year of life, [9] and the diagnosis is typically established only between two and four years of age [6,8]. |
| |
| Several studies have found macrocephaly to be overrepresented in PDD [4,6,9,11-19]. Leo Kanner, the investigator who first described the clinical features of PDD (1943), was also the first to notice increased head circumference (HC) in these patients [6,16-19]. Despite this, several studies found no association between PPD and macrocephaly [6,9]. There is also divergence in whether increased HC is [6,9,15] or is not [10,13,16,18] part of a broader spectrum of macrossomic features. Some investigators have found children with PDD to have a normal HC at birth [4,10,12,18]; others have even found such children to have lower values of HC at birth [10]. Gillberg [9] postulated that children with AS may have two different types of macrocephaly: one that is present from birth and one that emerges only after some years of development. Some studies found that HC growth was accelerated in the first year of life in children with AD [10,21] and PDD in general [6]. However the period within the first year in which the growth velocity was higher was not specified. Head circumference has been described by some authors to be normal in PDD in later adolescent and adult life [10-19]; Courchesne [10] described four stages in the growth velocity of HC: the first involves a slight undergrowth in the prenatal period, the second involves rapid and large overgrowth within the first year, the third appears to last about two to four years during which the overall rate slows, and the final stage involves a gradual decline in the rate of growth from middle or late childhood through to adulthood. |
| |
| There are some inconsistencies in investigations regarding the association between the growth velocity of HC and the severity of symptoms. Courchesne [10] found that earlier onset, a faster rate and longer period of excessive growth are related to more severe symptoms and poorer outcome; Mraz [6] also stated that a higher velocity of HC growth is associated with greater severity; Sacco [15] found that patients with larger head sizes appear more impaired in social and adaptation features but show an advantage in language development and general intelligence quotient measures, whereas patients with lower HC display less stereotypic behaviours but may be more neurologically impaired; Bailey [16] and Lainhart [18] found no association between HC size and cognitive function. |
| |
| The main objective of our study was to determine whether HC and the growth velocity of HC in children diagnosed with PDD are any different from children without neurodevelopmental disorders, at different age periods. |
| |
| Patients and Methods |
| |
| We designed an observational retrospective case-control study with prospective data collection based on the analysis of head circumference (HC) measures in 11 periods from birth to 36 months in two groups: 1) a study group of children diagnosed with pervasive development disorder (PDD) by a pediatric consultant based on the criteria outlined by DSM-IV (American Psychiatric Association, 1994) and with a follow-up in an outpatient clinic for development disorders in Hospital de Faro EPE; subjects were excluded for having evidence of a medical condition thought to be associated with PDD; 2) a control group of consecutive children attending general paediatric consultation at the hospital´s outpatient clinic, in which neurodevelopmental disorders were excluded by the attending physician, with at least five HC measures. HC data were collected from medical records (derived from the attending physician) of occipito-frontal circumference measures. The 11 periods considered corresponded to the regular medical consultations recommended by the Portuguese Ministry of Health: birth and months 1, 2, 4, 6, 9, 12, 15, 18, 24, 36. For each child we calculated the GV of HC (cm/month) for the first and second semester and for the first, second and third years of life. We also collected data related to gender, weeks of gestation and birth weight. |
| |
| The research was approved by Hospital de Faro Ethics Committee. Informed consent was obtained from all caregivers, both from the study and the control group. |
| |
| Given that medical records were unavailable for some patients and others did not include regular HC measures, our final study sample was smaller than the initial sample of patients that met the diagnostic criteria for PDD. We included in the study 90 children diagnosed with PDD, of which 32 were diagnosed with Autistic disorder (AD), 41 with Asperger disorder (AS) and 17 with pervasive development disorder not otherwise specified (PDD-NOS). The control group included 198 children. Not all children had records for all 11 HC measurements but we nevertheless used all the records available. |
| |
| Statistical analysis was performed using SPSS V16.0. The mean values of the different variables were compared using the Student t test. A multiple logistic regression model was used to test the influence of these variables on the diagnosis. Statistical significance was set at p≤0,05. R Software (R Development Core Team, 2008) was used to create the artwork. |
| |
| Results |
| |
| Our study sample included 90 children diagnosed with pervasive development disorder (PDD), of which, according to DSM-IV criteria, 32 corresponded to Autistic disorder (AD), 41 to Asperger disorder (AS) and 17 to pervasive development disorder not otherwise specified (PDD-NOS). The control group was composed of 198 children. Details regarding the study sample are given in Table 1. There was no significant difference between the PDD group and control group according to the duration of gestation (p=0.58) or birth weight (p=0.10). The gender was unequally distributed across groups, so statistical analysis was always performed separately by gender. Given the small number of females with AD and AS, we only included male gender analysis for these two subgroups; female population was only analysed altogether. |
| |
|
|
Table 1: Sample characteristics. |
|
| |
| In order to minimize possible confounding results regarding differences between groups in birth somatometric parameters, a multivariate regression was performed. Since weeks of gestation and weight at birth were correlated at a significance level of 0.01 (r=0.733), only the first was included in the logistic regression model. |
| |
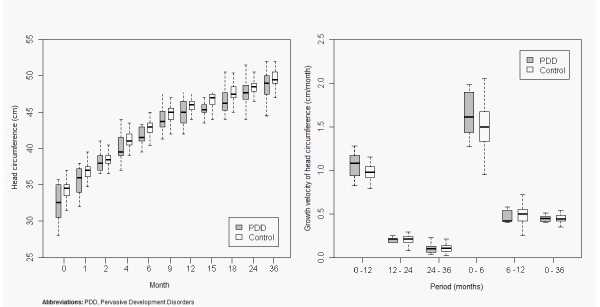
| Within the female population (Figure 1), we found sustained lower values of head circumference (HC) in the PDD compared to the control group, and this difference was statistically significant in several periods: birth (p=0.05) and months 4 (p=0.03), 6 (p=0.03), 9 (p=0.01), 12 (p=0.02), 15 (p<0.01) and 18 (p=0.02). |
| |
| |
|
|
Figure 1: Comparative analysis between girls with pervasive development disorder (PDD) and girls included in control group. |
|
| |
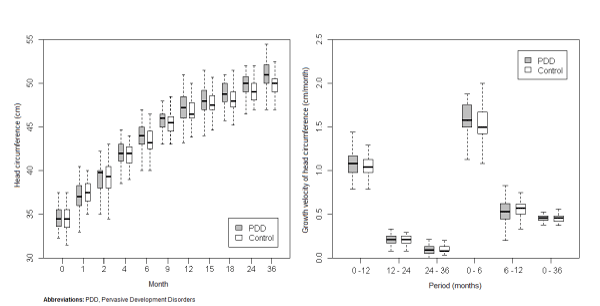
| In the comparative analysis between the total PDD male population and the control male group (Figure 2), we found sustained higher values of HC in the study group, although this was only statistically significant in one period (36 months, p=0.02). |
| |
|
|
Figure 2: Comparative analysis between boys with pervasive development disorder (PDD) and boys included in control group. |
|
| |
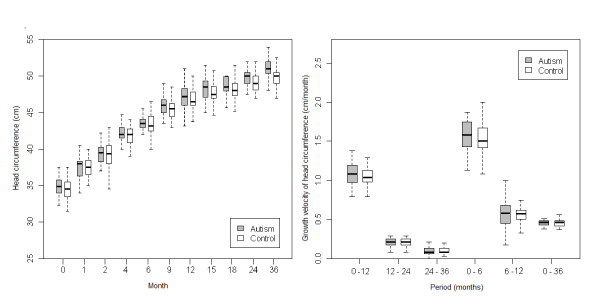
| The autistic disorder group had consistently higher values of HC when compared to the control group (Figure 3), although the differences were not significant in any period. |
| |
|
|
Figure 3: Comparative analysis between boys with autistic disorder and boys included in control group. |
|
| |
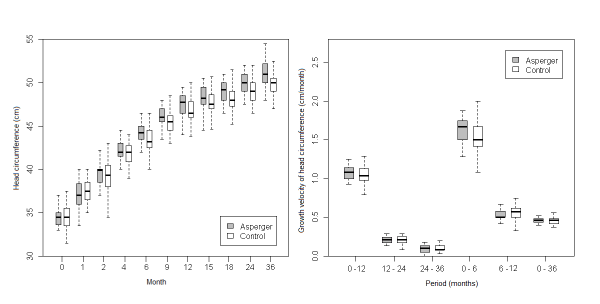
| More consistent results were found for higher values of HC in the AS group compared to the control group (Figure 4), where differences were statistically significant in six periods: months 6 (p<0.01), 9 (p=0.01), 12 (p=0.03), 18 (p=0.01), 24 (p=0.02) and 36 (p=0.02). |
| |
|
|
Figure 4: Comparative analysis between boys with Asperger disorder and boys included in control group. |
|
| |
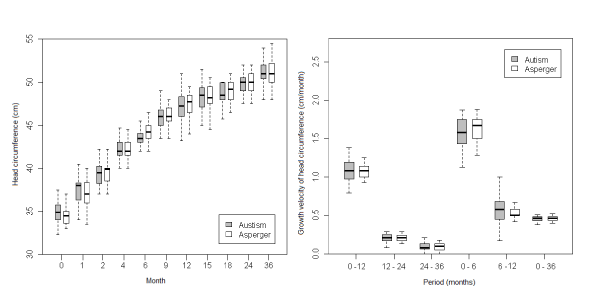
| In a comparative analysis of HC between AD and AS (Figure 5), although we found a trend for higher values of HC in the latter, differences were not significant except at 6 months (p=0.03). |
| |
|
|
Figure 5: Comparative analysis between boys with autistic disorder and boys with Asperger disorder . |
|
| |
| With regards to the growth velocity (GV) of HC, we found that it was higher for the PDD population (all female PDD, all male PDD, AS and AD) than the control group (Figures 1, 2, 3, 4, 5) in the first 6 months of life. Nevertheless, this difference was statistically significant only for AD (p=0.04). In addition there was a higher GV of HC in AD compared to the control group (p<0.01) for the periods 6 to 12 months and birth to 36 months. For the remaining periods, there were no significant differences in the GV of HC when comparing the PDD population to the control group and AD to AS. |
| |
| Discussion |
| |
| Pervasive development disorders (PDD) are thought to be a neurobiological condition, which implies that neurobiological abnormalities must precede the first behavioural expressions. There is evidence from neuropathological data for an evolving pathological process in the brain of children with PDD that extends from the foetal period of brain development into adulthood. Several studies have focused on the brain anatomy of patients with this disorder, based either on imaging (magnetic resonance imaging) or on histological data [10,12]. The results are inconsistent, probably due to the lack of statistical power resulting from small sample sizes, to the heterogeneity of the disorder itself or to the inability to control for potential confounding variables. The most consistent data however concern enlargement of both the grey and white matter volumes of the cerebral cortex [5,10,12]when compared to the control group; cerebellar grey and white matter enlargement has also been indicated [10]. Other studies have ascertained a higher overall cerebral volume in PDD children compared to healthy ones [5,10,12,13,15]. More pronounced enlargement of the grey matter has been encountered in low functioning autistic disorder (AD) compared to other PDD, and has therefore been related to a more severe clinical course [5]. |
| |
| The increase in brain volume could reflect either abnormal acceleration of cerebral growth or a failure of late prenatal and/or early postnatal regressive processes [10]. Gillberg hypothesized that there may be at least two different pathways to PDD, one connected with primary temporofrontal dysfunction (and late prenatal/early postnatal origins) and another linked to primary brainstem dysfunction (and early prenatal origins) [14]. The cellular basis of increased brain volume remains unclear and several hypotheses have been postulated: an excessive number or higher rates of growth of neurons and/or glial cells, excessive numbers of minicolumns, excessive and premature expansion of dendritic and axonal arbors, excessive numbers of axonal connections, smaller and more densely packed neurons in the cingulate gyrus and limbic system, premature myelination, and a reduction in the protein levels of the enzymes that synthesize g-aminobutyric acid and glutamic acid decarboxylase [5,10,14]. The rapid brain overgrowth at an early age that takes place in a critical period of brain development (marked by increases in synaptic connections, dendritic and axonal growth and myelination), must be an important factor in the emergence of characteristic behaviour. In such an important period of development of neuroplasticity and learning, aberrantly rapid and disordered growth without guidance may result in too many connections that may not be adaptive. |
| |
| The growth of the skull reflects the growth of the brain, and in the absence of gross abnormalities of skull shape, head circumference (HC) is a useful index of brain size and brain growth. As in our study, several other authors have used HC measures as a means of evaluation of brain volume. |
| |
| Within the male population, the higher values of HC obtained at birth in the PDD sample compared to the control group were not statistically different, in accordance with several other studies [4,10,12,18]. In agreement with the literature reviewed, we also found a tendency for higher HC values in PDD in other periods of the first three years of life, in the male gender [4,6,9,11-19]. This difference was evident when comparing the control group to PDD in general, to the AD group and to the Asperger disorder (AS) group. Nevertheless, statistical significance was attained in a sustained manner (in six of the 11 periods) only in the comparison between AS and the control group. Like several other authors [5,10], we also found a trend for higher values of HC in AS compared to AD in the first three years of life, although this difference was only statistically significant in one period (six months). |
| |
| In the male gender, a trend towards a higher growth velocity (GV) of HC in the first six months of life in PDD (PDD in general, AD and AS) was observed, although it was only statistically significant in AD. The growth velocity between six and 12 months and between birth and 36 months was also significantly higher in AD compared to the control group. For all other periods, and considering PDD in general, AD and AS, the growth velocity of HC did not differ significantly when compared to the control group. Likewise, we found no significant differences in the HC rate of growth when comparing AD and AS. This study thus indicates that it is the first six months in the first year of life during which the GV of HC appears to be more pronounced in children with PDD compared to the healthy population. |
| |
| To our knowledge, our study is the first to compare females diagnosed with PDD to a female control group. Given the small sample size of females diagnosed with AD and AS, we chose only to compare PDD in general to a control group. In this respect we found interesting results, as we observed a lower HC in every period of the first three years of life in the PDD group, most of which were statistically significant. As in males, we found higher velocities of HC growth in the first six months of life in the PDD group, although without statistical significance. |
| |
| Clinical symptoms are somewhat late to appear in PDD, and the diagnosis is more frequently established after the second year of life. Thereby there is a presymptomatic period during which neurodevelopmental abnormalities take place. In this period of greater plasticity of the brain, intervention may have more benefits in preventing, or at least ameliorating, symptoms. Therefore, it is crucial that earlier manifestations of the disorder are perceived. Increased HC may be such a manifestation and, if taken into consideration with other conditions, such as genetics and environmental risk factors, may increase the suspicion for the diagnosis. It should be emphasized however that increased HC may never be considered, per se, as a means of diagnosis for the disorder and should only be used as an additional factor for suspicion. |
| |
| Further research should be conducted on the identification of additional biological (such as biochemical, genetic and imaging data) and behavioural signs that could improve the accuracy and the timing of the diagnosis of PDD. In this way earlier implementation of therapeutic strategies and a more favourable clinical course for this serious and more and more prevalent disorder should be possible. |
| |
| With regards the limitations of our investigation we point to the heterogeneity of the control group and the eventual inclusion of undiagnosed cases of PDD in this group. However, we consider that the large sample size (of both the study and control groups) and the fact that HC measures were introduced by physicians unaware of their use in this investigation, minimized possible sources of bias. |
| |
| Conclusion |
| |
| In the present study we found differences related to head circumference in children diagnosed with Pervasive Development Disorders (PDD) when compared to a control group of children without neurodevelopmental disorders. We therefore emphasize the role of measuring head circumference in the evaluation of children with a suspected diagnosis of PDD. This somatometric parameter holds the potential for clinical application because its measurement is simple, inexpensive, noninvasive, rapid, accurate and reliable. |
| |
| |
| References |
| |
- Gillberg C (1995) Disorders of empathy: autism and autism spectrum disorders. In: Gillberg C (ed). Clinical Child Neuropsychiatry. Cambridge: Cambridge University Press 54-97.
- Gillberg C (2006) Autism spectrum disorders. In: Gillberg C, Harrington R, Steinhausen HC (ed). A Clinician´s Handbook of Child and Adolescent Psychiatry. Cambridge: Cambridge University Press 447-488.
- Dalton R, Forman MA, Boris NW (2004) Pervasive Developmental Disorders and Childhood Psychosis. In: Behrman RE, Kliegman RM, Jerson HB (ed). Nelson Textbook of Pediatrics 17th Edition. Philadepphia:WB Saunders Company 93-94.
- Caronna EB, Milunsky JM, Tager-Flusberg H (2008) Autism spectrum disorders: clinical and research frontiers. Arch Dis Child 93: 518-523.
- Lotspeich LJ, Kwon H, Schumann CM, Fryer SL, Goodlin-Jones BL, et al. (2004) Investigation of neuroanatomical differences between autism and Asperger syndrome. Arch Gen Psychiatry 61: 291-298.
- Mraz KD, Green J, Dumont-Mathieu T, Makin S, Fein D (2007) Correlates of head circumference growth in infants later diagnosed with autism spectrum disorders. J Child Neurol. 22:700-713.
- Dover CJ, Le Couteur A (2007) How to diagnose autism. Arch Dis Child 92: 540-545.
- Searching for early signs of autism spectrum disorders. World wide web electronic publication. https://www.health.harvard.edu
- Gillberg C, de Souza L (2002) Head circumference in autism, Asperger syndrome, and ADHD: a comparative study. Dev Med Child Neurol 44: 296-300.
- Courchesne E, Carper R, Akshoomoff N (2003) Evidence of brain overgrowth in the first year of life in autism. JAMA 290: 337-344.
- Conciatori M, Stodgell CJ, Hyman SL, O'Bara M, Militerni R, et al. (2004) Association between the HOXA1 A218G polymorphism and increased head circumference in patients with autism. Biol Psychiatry 55: 413-419.
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, et al. (2005) Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry 62: 1366-1376.
- Torrey EF, Dhavale D, Lawlor JP, Yolken RH (2004) Autism and head circumference in the first year of life. Biol Psychiatry 56: 892-894.
- Palmen SJ, van Engeland H, Hof PR, Schmitz C (2004) Neuropathological findings in autism. Brain 127: 2572-2583.
- Sacco R, Militerni R, Frolli A, Bravaccio C, Gritti A, et al. (2007) Clinical, morphological, and biochemical correlates of head circumference in autism. Biol Psychiatry 62: 1038-1047.
- Woodhouse W, Bailey A, Rutter M, Bolton P, Baird G, et al. (1996) Head circumference in autism and other pervasive developmental disorders. J Child Psychol Psychiatry 37: 665-671.
- Fidler DJ, Bailey JN, Smalley SL (2000) Macrocephaly in autism and other pervasive developmental disorders. Dev Med Child Neurol 42: 737-740.
- Lainhart JE, Piven J, Wzorek M, Landa R, Santangelo SL, et al. (1997) Macrocephaly in children and adults with autism. J Am Acad Child Adolesc Psychiatry 36: 282-290.
- Wallace GL, Treffert DA (2004) Head size and autism. Lancet 363: 1003-1004.
- Kanner L (1968) Autistic disturbances of affective contact. Acta Paedopsychiatr 35: 100-136.
- Dawson G, Munson J, Webb SJ, Nalty T, Abbott R, et al. (2007) Rate of head growth decelerates and symptoms worsen in the second year of life in autism. Biol Psychiatry 61: 458-464.
- Vale MC, Pinto M (2009) Perturbações do espectro do autism. In: Amaral JMV, eds. Tratado de Clínica Pediátrica. 1ª edição. Lisboa 154-161.
- Idiazábal-Aletxa MA, Boque-Hermida E (2007) [Cognitive processing in autism spectrum disorders]. Rev Neurol 44 Suppl 2: S49-51.
- Etchepareborda MC (2005) Funciones ejecutivas y autismo. Rev Neurol. 41: S155-S162.
- Allen DA, Steinberg M, Dunn M, Fein D, Feinstein C, et al. (2001) Autistic disorder versus other pervasive developmental disorders in young children: same or different? Eur Child Adolesc Psychiatry 10: 67-78.
- Ehlers S, Gillberg C, Wing L (1999) A screening questionnaire for Asperger syndrome and other high-functioning autism spectrum disorders in school age children. J Autism Dev Disord 29: 129-141.
- Myhr G (1998) Autism and other pervasive developmental disorders: exploring the dimensional view. Can J Psychiatry 43: 589-595.
- Walker DR, Thompson A, Zwaigenbaum L, Goldberg J, Bryson SE, et al. (2004) Specifying PDD-NOS: a comparison of PDD-NOS, Asperger syndrome, and autism. J Am Acad Child Adolesc Psychiatry 43: 172-180.
- Ayuda-Pascual R, Martos-Pérez J (2007) [The influence of the social perception of emotions in the formal language of children with Asperger's syndrome or high-functioning autism]. Rev Neurol 44 Suppl 2: S57-59.
- Cox AD (1991) Is Asperger's syndrome a useful diagnosis? Arch Dis Child 66: 259-262.
- Hill EL, Frith U (2003) Understanding autism: insights from mind and brain. Philos Trans R Soc Lond B Biol Sci 358: 281-289.
- Belinchón-Carmona M, Posada-De la Paz M, Artigas-Pallarés J (2005) Guía de buornos del espectro autista. Rev Neurol. 41: 371-377.
- Poirier N, Forget J (1998) [Diagnostic criteria of autism and Asperger's syndrome: similarities and differences]. Sante Ment Que 23: 130-148.
- Ayuda-Pascual R, Martos-Pérez J (2007) [The influence of the social perception of emotions in the formal language of children with Asperger's syndrome or high-functioning autism]. Rev Neurol 44 Suppl 2: S57-59.
- Dean CB, Nielsen JD (2007) Generalized linear mixed models: a review and some extensions. Lifetime Data Anal 13: 497-512.
|
| |
| |