| Rapid Communication |
Open Access |
|
| Xia Tang1,2, Zhi Dang1,4*, Li-Yuan He1, Gui-Ning Lu1, Xue-Qin Tao3 and Xiao-Yun Yi1,4 |
| 1School of Environmental Science and Engineering, South China University of Technology, Guangzhou Higher Education Mega Center, Guangzhou 510006, P.R. China |
| 2Guangzhou sewage treatment CO.,LTD., Guangzhou 510163, P.R. China |
| 3School of Environmental Science and Engineering, Zhongkai University of Agriculture and Engineering, Guangzhou 510225, P.R. China |
| 4The Key Lab of Pollution Control and Ecosystem Restoration in Industry Clusters, Ministry of Education, Guangzhou 510006, P.R. China |
| *Corresponding author: |
Zhi Dang
School of Environmental Science and Engineering
South China University of Technology
Guangzhou Higher Education Mega Center
Guangzhou 510006, P.R. China
Tel: +86-20-39380522
Fax: +86- 20-39380569
E-mail: chzdang@scut.edu.cn. |
|
| |
| Received March 10, 2012; Published June 23, 2012 |
| |
| Citation: Tang X, Dang Z, He LY, Lu GN, Tao XQ (2012) Biodegradation of Crude Oil by an Artificial Microalgal-Bacterial Consortium. 1: 118. doi:10.4172/scientificreports.118 |
| |
| Copyright: © 2012 Tang X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Degradation process of crude oil by one artificial microalgal-bacterial consortium was described in this study. The consortium was constructed by one axenic Scenedesmus obliquus named GH2 and four oil component-degrading bacteria with known complementary degradative capabilities, including Sphingomonas GY2B, Burkholderia cepacia GS3C, Pseudomonas GP3A and Pandoraea pnomenusa GP3B. Results showed the consortium could completely eliminate alkanes, alkylcycloalkanes and alkylbenzens in 10 days, 7 days and 7days, respectively. No transformation in the composition of the saturated hydrocarbons upon the consortium growth. The consortium preferential attacked high molecular Polyaromatic Hydrocarbons (PAHs) such as phenanthrene and methylphenanthrenes, a lot of C2, C3 naphthalene isomers and some extra lower molecular substances were produced during the PAHs degradation. The total biodegradation rates of naphthalene, fluorene, phenanthrene and their derivatives were achieved about 90%, 76% and 70%, respectively. The population dynamics of the microorganisms showed the degradation process closely correlated with the growth of bacterial strains. |
| |
| Keywords |
| |
| Algal-bacterial consortium; Crude oil; Degradation process; Population dynamics |
| |
| Introduction |
| |
| The extensive use of petroleum and petroleum products leads to serious oil contamination of the environment, thus considerable interests were taken in microbial degradation and detoxification of these pollutants. Microalgae play an important role in the environmental cleaning of pollutants. Microalgae provide O2 to aerobic bacteria to mineralize pollutants and then take-up the released CO2 [1]. Furthermore, microalgae can help in co-metabolic degradation or produce biosurfactants and extracellular matters to enhance bacterial activity for increasing pollutant bioavailability [2]. Additionally, microalgae can accumulate hydrocarbons, and subsequently make those compounds available to the associated hydrocarbon-utilizing bacteria [3]. The cooperation of phototrophic microalgae and heterotrophic bacteria could potentially improve the degradation of environmental contaminants including oil pollutants [4,5]. |
| |
| Petroleum products contain thousands of individual hydrocarbons and related compounds. Their main components are usually subdivided into saturates (n- and branched- chain alkanes and alkylcycloalkanes ) and aromatics (monoaromatic hydrocarbons and polyaromatic hydrocarbons). The less abundant resins and asphaltenes consist of more polar compounds, containing heterocycles, oxygenated hydrocarbons and aggregates with high molecular weight [6]. Given the complexity of oil pollutants, however, it is hard to find a microorganism species that can breakdown a mixture of pollutants completely [7], one microorganism can only degrade a limited number of crude oil components [8]. Oil-degrading mixed cultures with broad enzymatic capabilities would be required to achieve extensive degradation. Thus, an artificial microalgal-bacterial consortium was constructed by one axenic Scenedesmus obliquus and four oil-component degrading bacteria with known complementary degradative capabilities [9]. |
| |
| The present study was focus on the degradation process by this artificial microalgal-bacterial consortium. The main components of crude oil, saturates (alkanes and alkylcycloalkanes) and aromatics (alkylbenzenes and PAHs) were investigated respectively. Denaturant Gradient Gel Electrophoresis (DGGE) was used to monitor the population dynamics of the consortium, in order to elucidate the metabolic capabilities in correlation to the growth of the constitutors. |
| |
| Materials and Methods |
| |
| Microorganisms and culture conditions |
| |
| BG11 medium was used throughout all the experiments. The composition was as follows [10]. (g.L-1 in distillated water): 1.5 NaNO3, 0.04 K2HPO4•3H2O, 0.075 MgSO4•7H2O, 0.036 CaCl2•2H2O, 0.006 citric Acid 0.006 ferric ammonium citrate; 0.001 EDTA, 0.02 Na2CO3; 6.1x10-5 H3BO3; 1.69x10-4 MnSO4•H2O; 2.87x10-4 ZnSO4•7H2O, 2.5x10-6 CuSO4•5H2O and 1.25x10-5 (NH4)6Mo7O24•4H2O. |
| |
| The artificial microalgal-bacterial consortium formed by one axenic Scenedesmus obliquus named GH2 and four oil component-degrading bacteria. The axenic Scenedesmus obliquus exhibited no oil degrading ability; but significantly promoted the degradation ability of other oil component-degrading bacteria [9]. The bacterial strains included Sphingomonas GY2B (Genbank accession number DQ139343) [11,12] and Burkholderia cepacia GS3C (Genbank accession number EU282110) [13] along with a mixed culture named GP3 containing Pseudomonas GP3A (Genbank accession number EU233280)and Pandoraea pnomenusa GP3B (Genbank accession number EU233279) [14]. GY2B could only degrade polyaromatic hydrocarbons; GS3C was able to degrade aliphatic chain hydrocarbons, and GP3 could utilize both saturates and aromatic hydrocarbons [9] 2 ml (chlorophyll content was 2.62 μg/ml) algae culture and 1ml mixed (v:v:v = 1:1:1, 1.0 × 107 cells/ml bacteria culture were initial inoculated into 30 ml BG11 medium for the consortium construction, and the consortium culture were transferred into fresh BG11 medium once every 7days. |
| |
| In the biodegradation study, 3ml microalgal-bacterial cultures were inoculated into100 ml flasks containing 30 ml BG11 medium. Oseberg crude oil was added as the initial concentration was 0.3% (v/v). Flasks were incubated at 25 ± 1°C with constant shaking at 150 rpm under a light-dark regime of 14:10 (illumination: 165 μmol photons/m2•s) by cool white fluorescent tubes (Opple, T5). Samples were taken on the 0 (original), 2, 3, 4, 5, 7 and 10 day, respectively, for chemical analysis, and all assays were carried out in triplicate. |
| |
| Chemical Analysis |
| |
| The residual oil was recovered from the culture with n-hexane. Biomass was removed by centrifugation and the supernatant oil recovered with the addition of 15 ml n-hexane, oil adhering to the microalgae or the flask was extracted ultrasonically with another 15 ml n-hexane for 30 minutes, these two volumes were combined to a total of 30 ml final volume. All the extracts were fractioned into four parts including saturates, monoaromatics, polyaromatics and polars, the saturated and aromatic hydrocarbons were analyzed by GC/MS as described by [9]. |
| |
| Population dynamics analysis |
| |
| For the biodegradation assays with the microalgal-bacterial consortium, microorganism samples were obtained at every sampling time. Cells were harvested by centrifugation and the biomass resuspended in TE (Tris-HCl 10mM, EDTA1mM, pH 8) buffer. DNA was extracted from 2ml suspensions using the lysis/phenol method [15]. 0.5μl genomic DNA (60~100 ng/μl) of each sample was used as template for PCR, the 16S rRNA gene fragment was amplified with the primers 358F-GC and 907R [15]. |
| |
| DGGE was run in a DCode system (Bio-Rad), with a 6% polyacrylamide gel, and the gradient concentrations were 30% to 60%. The mixture (100 μl) of two replicated PCR products was loaded for each sample, the gel was electrophoresized at 80V for 15h at 60°C and then silver-stained. The DNA recovered from excised DGGE bands were reamplified and sequenced by the Beijing Genomics Institute (China). |
| |
| Results and Discussion |
| |
| Biodegradation process |
| |
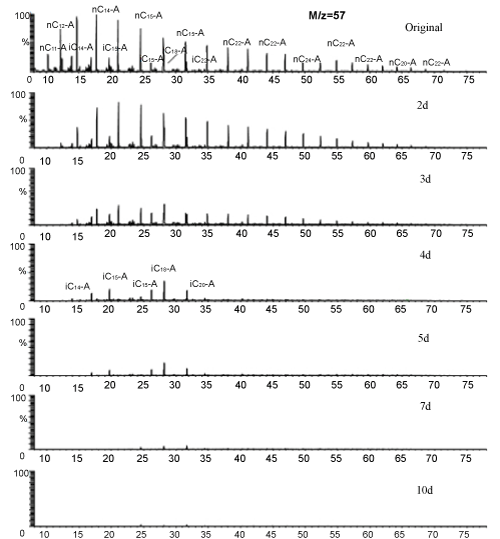
| Degradation processes of alkanes by the artifitial microalgalbacgterial consortium were showed in Figure 1. n-Alkanes had been completely removed in 4 days, while the group of isoprenoids was unchanged except slightly decrease of i-C14 alkane. The more recalcitrant of isoprenoids for biodegradation would be attributed to the branched chemical structure, the presence of n-alkanes also made repressed action for i-alkanes degradation [6]. No n-alkanes presented and most of i-alkanes decreased since the 4th day. The group of i-alkanes could also been eliminated in 10 days. |
| |
|
|
Figure 1: Biodegradation process of alkanes. nCx: straight-chain alkanes, iCx: branched alkanes. |
|
| |
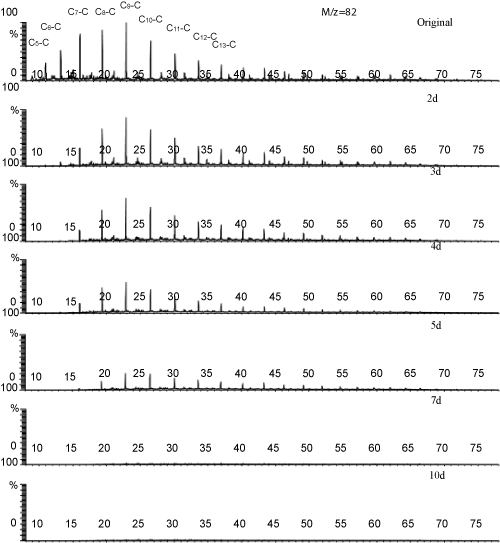
| From the Figure 2, it can be seen that the homologues of alkylcycloalkane up to C9 were began to degrade in the 2nd day, the C5 derivatives was eliminated and only a few C6 derivatives retained, but the homologues more than C10 remained constantly. The long chainalkylcycloalkanes decreased continuously since the 3rd day. Although cycloalkanes were reported as more recalcitrant oil components for biodegradation [16], the artificial consortium could removed all of these homologues in 7days. Perry [17] reported isoalkanes were preferentially degraded than cycloalkanes, but that was not strictly valid in this study. No transformation in the composition of the saturated hydrocarbons upon the consortium growth. |
| |
| |
|
|
Figure 2: Biodegradation process of alkylcycloalkanes Cx-C: alkylcycloalkanes. |
|
| |
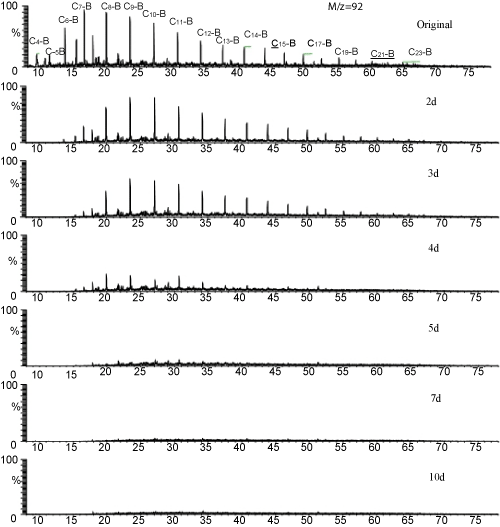
| In the 2nd day, the artificial consortium could attacke the homologues of alkylbenzene up to C9, C4 and C5 derivatives were eliminated, and the homologues more than C10 kept unchangeably. The C10 homologues begin to degrade and C7 homologue was removed in the 3rd day, but the homologues higher than C11 still remained constantly. The long-chain homologues (with carbon numbers more than C12) dropped abruptly between the 3rd and the 4th day, most of these substances completely removed during this one day’s period. All of these homologues were completely depleted in 7 days (Figure 3). |
| |
|
|
Figure 3: Biodegradation process of alkylbenzenes Cx-B: alkylbenzenes. |
|
| |
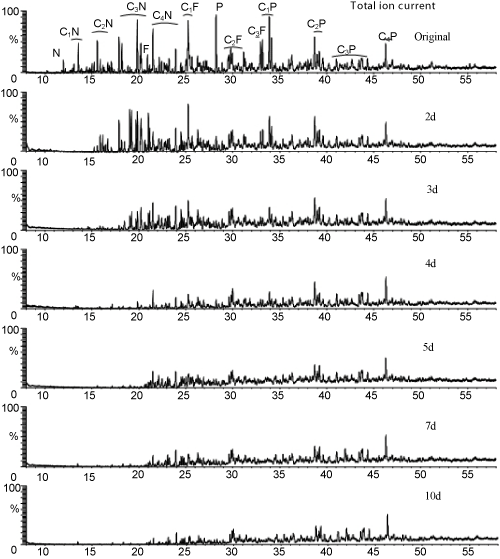
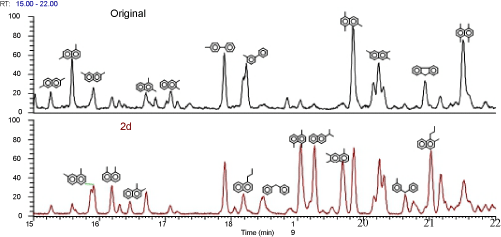
| Figure 4 showed the mass spectrometric total ion current of the control and biodegraded PAHs. Phenanthrene and the methylphenanthrenes were degraded about 90% and 50%; fluorene was eliminated and the C1, C2 derivatives were decreased about 45%and 35%, respectively; naphthalene was eliminated and C4- naphthalenes derivatives were depleted about 10% in the 2nd day. A lot of C2, C3 naphthalene isomers and some extra substances, such as 2-methyl-1-propylnaphthalene, 1-propylnaphthalene, 1-benzyl-3- methylbenzene and diphenylmethane were augmentations in the 2nd day’s ion current (Figure 5). That suggested in crude oil mixtures, the carbochain and benzene ring of high molecular PAHs may be broken directly during the degradation process. Such as methylphenanthrenes may attaked by breaking one benzene ring firstly as 2-methyl-1 propylnaphthalenes were the intermediate products, the fracture of the alkyl chains then transform 2-methyl-1propylnaphthalenes into C2, C3 naphthalene isomers. C4 naphthalene may also convert to C2, C3 naphthalene isomers by demethylation. 1-benzyl-3-methylbenzene and diphenylmethane might be attributed to partially fracture of fluorene and their derivatives. Although a great deal work had been done on the pathway of oil components degradation, most studies were carried out by choosing one component as typical substance [12], the pathways of each component in bulk crude oil degradation have not yet been clearly delineated since there were too many complex mixture substances. Essentially, it is hard to compare the degradation pathway of one component in crude oil with the same compound as single pure substance, because of the mutual effect between components; the intermediate products may also affect the degradation of other component. Extensive research should be carried out on this topic. |
| |
|
|
Figure 4: Mass spectrometric total ion current showing the original and biodegraded PAHs of crude oil Cx-N: alkylated naphthalenes, Cx-F: alkylated fluorenes, Cx-P: alkylated phenanthrenes. |
|
| |
|
|
Figure 5: The composition changes of PAHs in the 2nd day. |
|
| |
| In the 3rd day, the C2 naphthalene derivatives were eliminated, more than half of residual C3, C4 naphthalenes were depleted; C1 and C3 homologues of fluorene were decreased to around 40% and 10% of the initial quantity; phenanthrene was eliminated and about 30% and 40% of C1 and C2 phenanthrene were decreased; no additional peak emerged in the 3rd day’s mass spectrogram. C2 fluorene were decreased to about 50% in the 4th day, other substances also decreased except C3 and C4 phenanthrene, which remains constant throughout the degradation process. |
| |
| The major degradation of PAHs occurred in 5 days, no obvious difference between mass spectrum current of the 5th day and 10th day expect slight decrease of low molecular materials. The residual oil contained a small quantity of C4 naphthalene, C1 and C2 fluorene, C2 phenanthrene. C3 and C4 phenanthrene are biorefractory and couldn’t be degraded absolutely. The total biodegradation rates of naphthalene, fluorene, phenanthrene and their derivatives were achieved about 90%, 76% and 70%, respectively. |
| |
| Microorganisms’ population dynamics |
| |
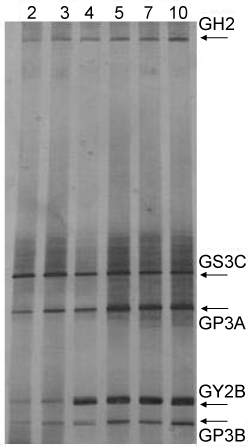
| To determine the microorganisms’ population dynamics during the biodegradation processes of crude oil, DGGE-profiles of the consortium were obtained at different culturing times (Figure 6). GS3C and GP3A showed intense bands since the 2nd day, the brightness and density of GY2B rose sharply in the 4th day, while the band corresponding to GH2 and GP3B increased gradually during the culturing times. |
| |
|
|
Figure 6: DGGE fingerprints of the consortium determined for crude oil biodegradation assay. Sampling time (days) for each assay were indicated in the figure. |
|
| |
| GS3C was more efficient in degradation of aliphatic chain hydrocarbons. GP3 (containing GP3A and GP3B could attack the aliphatic hydrocarbons but also degraded the aromatic hydrocarbons. GP3A belongs to Pseudomonas, which was reported as the most important genus of hydrocarbon utilizers [18]. GY2B was specialized and the most efficient in the degradation of PAHs [9] According to the DGGE banding patterns, GS3C and GP3A were predominated and mainly responsible for the aliphatic hydrocarbons degradation since the 2nd day. The growth of GP3B achieve the stationary phase in the 5th day,mixed culture of GS3C and GP3 would significantly promoted the degradation of alkylcycloalkanes [9] and the homologues of alkylcycloalkane were eliminated during the 5th to the 7th day (Figure 2). GY2B were initially isolated by using phenanthrene as sole carbon source [11] and it has reserved preferential degradation property for the high molecular PAHs such as phenanthrene and methylphenanthrenes. The extensive decrease of PAHs leaded to sharp increase of GY2B in the 4th day. The cooperation of GS3Cand GY2B would cause co-oxidation of alkylbenzenes [9] the intensive growth of GY2B thuslead the longchain alkylbenzenes homologues dropped abruptly between the 3rd and the 4th day (Figure 3). The microorganisms’ population dynamics showed the degradation process closely correlated with the growth of bacterial strains and their metabolic activity. |
| |
| Acknowledgements |
| |
| The work was financially supported by the National Natural Science Foundation of China (No. 40730741), the Guangdong Provincial Science and Technology Planning Project (No.2007A050100023), the Guangdong Provincial Natural Science Foundation (No. 9351064101000001), the Fundamental Research Funds for the Central Universities (Nos. 2009ZM0034 and 2009ZM0119) and the Open Foundation of the State Key Lab of Environmental Geochemistry. |
| |
| |
| References |
| |
- Oswald WJ (1988) Micro-algae and waste-water treatment. In: Borowitska MA, Borowitzka LJ(ed) Micro-algal Biotechnology. Cambridge 305-328.
- Muñoz R, Guieysse B, Mattiasson B (2003) Phenanthrene biodegradation by an algal-bacterial consortium in two-phase partitioning bioreactors. Appl Microbiol Biotechnol 61: 261-267.
- Radwan SS, Al-Hasan RH, Ali N, Salamah S, Khanafer M (2005) Oil-consuming microbial consortia floating in the Arabian Gulf. Int Biodeterior Biodegradation56: 28-33.
- Safonova ET, Dmitrieva IA, Kvitko KV(1999) The interaction of algae with alcanotrophic bacteria in black oil decomposition. Resources Conservation and Recycling 27: 193-201.
- Chaillan F, Gugger M, Saliot A, Coute A, Oudot J (2006) Role of cyanobacteria in the biodegradation of crude oil by a tropical cyanobacterial mat. Chemosphere 62: 1574-1582.
- Abalos A, Viñas M, Sabaté J, Manresa MA, Solanas AM (2004) Enhanced biodegradation of Casablanca crude oil by a microbial consortium in presence of a rhamnolipid produced by Pseudomonas aeruginosa AT10. Biodegradation 15: 249-260.
- Alxander M (1994) Biodegradation and bioremediation. California, San Diego.
- Braggs JR, Prince RC, Harner EJ, Atlas RM (1994) Effectiveness of bioremediation for the Exxon Valdez oil spill. Nature 368: 413-418.
- Tang X, He LY, Tao XQ, Dang Z, Guo CL, et al. (2010) Construction of an artificial microalgal-bacterial consortium that efficiently degrades crude oil. J Hazard Mater 181: 1158-1162.
- Rippka R (1989) Methods in enzymology. California, San Diego.
- Tao XQ, Lu GN, Dang Z, Yang C, Yi XY (2007) A phenanthrene-degrading strain Sphingomonas.sp GY2B isolated from contaminated soils. Process Biochem 42: 401-408.
- Tao XQ, Lu GN, Dang Z, Yi XY, Yang C (2007) Isolation of phenanthrene-degrading bacteria and characterization of phenanthrene metabolites. World J Microbiol Biotechnol23: 647-654.
- Wu RR, Dang Z, Yi XY, Yang C, Chen XP (2009) Effect of amino acids on degrading capability of a n-alkanes degrading strain GS3C. Res Environ Sci 22: 702-706.
- Chen X, Yi X, Tao X, Wu R, Yang C, et al. (2008) Screening and characterization of pyrene-degrading microbial. Chinese Journal of Environmental Engineering2: 413-417.
- Sánchez O, Diestra E, Esteve I, Mas J (2005)Molecular Characterization of an Oil-Degrading Cyanobacterial Consortium. Microb Ecol 50: 580-588.
- Sugiura K, Ishihara M, Shimauchi T, Shigeaki H (1996) Physicochemical properties and biodegradability of crude oil. Environ Sci Technol31: 45-51.
- Perry JJ (1984) Microbial metabolism of cyclic alkanes. In: Atlas RM (ed) Petreum Microbiology. Macmillan Publi Co, New York 61-98.
- Bartha R (1977) The microbiology of aquatic oil spills. Adv Appl Microbiol 22: 225-266.
|
| |
| |