| Research Article |
Open Access |
|
| P. Karthic*, Shiny Joseph and Naveenji Arun |
| Department of Chemical Engineering, National Institute of Technology Calicut, Kozhikode, India |
| *Corresponding authors: |
P. Karthic
National Institute of Technology Calicut
Kozhikode - 673601, India
Tel: 0495 2520950
Fax: 0495 2287250
E-mail: apkarthic@nitc.ac.in |
|
| |
| Received February 02, 2012; Published July 30, 2012 |
| |
| Citation: Karthic P, Joseph S, Arun N (2012) Optimization of Process Variables for Biohydrogen Production from Glucose by Enterobacter aerogenes. 1: 173. doi:10.4172/scientificreports.173 |
| |
| Copyright: © 2012 Karthic P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| The individual and interactive effects of significant process parameters were investigated for the optimization of biohydrogen production using glucose as a substrate. Response surface methodology was applied to optimize the process parameters for maximum hydrogen production using Enterobacter aerogenes MTCC 111. The important factors influencing hydrogen production such as glucose, initial pH, inoculum size, tryptone, yeast extract, and ferric chloride were screened using Plackett-Burman design. Based on the Plackett-Burman design, significantly influencing process variables identified were glucose, initial pH and ferric chloride. 3-dimensional (3-D) response surface and 2-dimensional (2-D) contour analysis were adopted to further investigate the mutual interaction between the parameters and to determine the optimal values for maximum hydrogen yield. The optimal values estimated using the statistical design to achieve maximum H2 yield of 1.69 mol H2/mol glucose were glucose 16.56 g/l, initial pH 6.15 and ferric chloride 213.13 mg/l. |
| |
| Keywords |
| |
| Biohydrogen; Modified gompertz model; Box-Behnken design; Enterobacter aerogenes; Dark fermentation. |
| |
| Introduction |
| |
| Biological hydrogen production is an eco-friendly, harmless process carried out under mild operating conditions, using renewable sources as substrates. Fermentative hydrogen production is a very complex process and is influenced by many factors. Clostridium butyricum and Enterobacter aerogenes have been known to be strong and efficient producers of hydrogen [1]. Enterobacter aerogenes is a representative of facultative anaerobes can rapidly consume oxygen and recover the activity of Fe-hydrogenase under anoxic condition in contrast to strict anaerobes which are sensitive to oxygen inhibition. The disadvantage of the dark fermentative process is its lower achievable yield compared to photosynthetic route, appears too low to be economically attractive as an alternative to the existing conventional methods. It was reported that the conversion of pyruvate to solvent and acids were the main reasons for obtaining lower yields than the theoretical value [2]. Microorganisms are capable to change their metabolic pathway according to metabolites (volatile fatty acids) concentration which is greatly influenced by environmental factors such as initial pH, temperature and nutritional requirements [3]. The optimization of nutritional and environmental conditions plays a vital role in developing bioprocesses and improving their performance [4]. It is very tedious and time-consuming to perform the operation using one-factor-at-a- time method [5]. This method may lead to unreliable results and inaccurate conclusions. Moreover, it does not depict the interactive effects among the variables and guarantee the determination of optimal conditions. On the other hand, the statistically based experimental design is a time-saving method, which minimizes the error in determining the interactive effect of process parameters [6]. Statistical optimization design on biohydrogen production has recently been reported in literatures [7-9]. Optimization studies were carried out using Enterobacter aerogenes with respect to hydrogen production rate [10-12]. Some studies proved that the process parameters such as pH, temperature and iron concentration had significant influence on biohydrogen production [13,14]. Although many studies have been done on the effect of various environmental factors on hydrogen production, the information on the statistical optimization of factors such as yeast extract, tryptone and ferric chloride using Enterobacter aerogenes are still lacking. Therefore, this present study aims to investigate the parameters significance and optimize the nutritional and environmental factors affecting hydrogen production from glucose using Enterobacter aerogenes. |
| |
| Materials and Methods |
| |
| Micro-organism and pre-cultivation |
| |
| Facultative anaerobe Enterobacter aerogenes MTCC 111 was obtained from Microbial type culture collection, Chandigarh. Pure culture of the cells was maintained on nutrient agar slants at 4°C and sub-cultured once in a month. |
| |
| Experimental procedure |
| |
| All batch experiments were conducted in 250 ml conical flask. 1 L of synthetic medium was prepared at various pH values, glucose concentrations (g/l) and iron concentrations (mg/l). The synthetic medium consisted of (in g/l): KH2PO4, 0.75; K2HPO4, 0.75; MgSO4.6H2O, 0.8; MnSO4.4H2O, 0.2; sodium chloride, 0.2; yeast extract, 4; (NH4)2SO4, 2; L-cysteine hydrochloride monohydrate, 0.5 in the conical flask. The initial pH of the medium was adjusted using 3N HCl and/or 3N NaOH solution. The room temperature (30°C) was maintained throughout the batch experiment. The flasks were then flushed with nitrogen gas before the start up to remove oxygen in the headspace of the flasks to ensure anaerobic condition. These flasks were immediately air-sealed with butyl rubber stopper and covered with aluminium seal cap. The flasks were covered with aluminium foil to prevent the contact of sunlight. These were agitated in an orbital shaker kept at 120 rpm to provide better contact among the components. The evolved gas was collected and determined by the water displacement method in graduated cylinders prefilled with water which was adjusted to pH 3.0 or less in order to prevent dissolution of the gas [15]. Each batch test was conducted in triplicate and their average was taken. |
| |
| Analytical methods |
| |
| Hydrogen gas generated during experiments was estimated using a microprocessor based pre-calibrated H2 sensor (electrochemical sensor, ExTox Gas Detector 4–20 mA version, GmBH Inc., Germany) [16]. The output signal displayed % volume of H2 in the headspace of flasks, which was further converted to mmol. The sensor has a measuring range of 0–30% H2 with 5s response time in a temperature range of 20–50°C. The system was calibrated once in a week using calibration cap provided with the instrument. pH values were determined by a pH meter (Systronic Instruments Ltd., India). |
| |
| Response surface methodology |
| |
| Plackett–Burman design: A 2k factorial Plackett–Burman design [17] is used to reduce the number of ingredients and the medium components required for the production of hydrogen and are given based on the first order model: |
| |
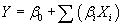 (1) (1) |
| |
where, Y is the response (maximum H2 yield), 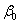 is the model intercept and is the model intercept and 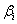 is the linear coefficient and Xi is the level of the independent variable. Each factor in the design was prepared in two levels: -1 for low level and +1 for high level and screened in twelve experimental designs. Table 1 illustrates the levels of each factor and their statistic analysis in the experimental design. Experiments were conducted based on the Plackett-Burman design and the corresponding H2 yield was presented in the Table 2. is the linear coefficient and Xi is the level of the independent variable. Each factor in the design was prepared in two levels: -1 for low level and +1 for high level and screened in twelve experimental designs. Table 1 illustrates the levels of each factor and their statistic analysis in the experimental design. Experiments were conducted based on the Plackett-Burman design and the corresponding H2 yield was presented in the Table 2. |
| |
|
|
Table 1: Levels and statistic analysis of variables for Plackett-Burman Design. |
|
| |
|
|
Table 2: Evaluation of variables influencing hydrogen yield using Plackett-Burman design. |
|
| |
| Box-Behnken design: In order to optimize the critical factors for enhanced hydrogen production, a three-variable Box–Behnken design [18] with three replicates at the centre point was applied. For statistical calculations, the relation between the coded and actual values are used and is given by |
| |
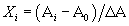 (2) (2) |
| |
| Where Xi is a coded value of the variable; Ai the actual value of variable; A0 the actual value of the Ai at the centre point; and ΔA, the step change of variable. Based on the Box–Behnken design, a minimum of 15 combinations of all the three factors including 3 replicates at the centre point was prepared and the experimental design with respective H2 yield obtained was represented in Table 3. For predicting the optimal condition, the quadratic polynomial equation was fitted to correlate the relationship between variables and response (i.e., hydrogen yield), and estimated with the following equation: |
| |
|
|
Table 3: The Box-Behnken experimental design with three independent variables. |
|
| |
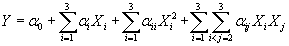 (3) (3) |
| |
Where Xi are the input variables, which influence the response variable Y; 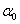 is the offset term; is the offset term;  is the ith linear coefficient; is the ith linear coefficient; 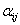 is the ijth interaction coefficient. The input values of X1, X2 and X3 corresponding to the maximum value of Y were solved by setting the partial derivatives of the functions to zero. is the ijth interaction coefficient. The input values of X1, X2 and X3 corresponding to the maximum value of Y were solved by setting the partial derivatives of the functions to zero. |
| |
| Kinetic analysis |
| |
| The modified Gompertz equation (Equation (4)) was used to determine the cumulative hydrogen production [19]. |
| |
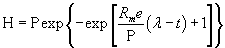 (4) (4) |
| |
| Where, H is the cumulative volume of hydrogen produced (mL), Rm is the maximum hydrogen production rate (mL H2/lh), λ is the lag-phase time (h), t is the incubation time (h), P is the hydrogen production potential (mL H2) and e is 2.718. Parameters (P, Rm and λ) were determined by best fitting the hydrogen production data for Equation (4) using the Matlab 7.3.0 (R2006b) version with curve fitting toolbox [20]. H2 yield could be determined by dividing the cumulative hydrogen produced by the amount of glucose added. |
| |
| Results and Discussion |
| |
| Screening of culture parameters |
| |
| The combined effect of initial pH, glucose, peptone, yeast extract, tryptone and ferric chloride for hydrogen production were investigated using Plackett–Burman design. In the Table 2, the main effect of each variable upon hydrogen yield was estimated as the difference between both averages of measurements made at the high level (+1) and at the low level (-1) of that factor. The positive sign of the effect, Exi of the tested variable implies that the influence of the variable on hydrogen yield is greater at a high level; the negative sign shows that the influence of the variable is greater at a low level. From the multiple linear regression analysis, it was observed that the main effect and the corresponding t-values are negative for the variables X3 (inoculum size), X4 (yeast extract) and X5 (tryptone), whereas positive for X1 (glucose), X2 (initial pH) and X6 (ferric chloride) (Table 1). Variables X3, X4 and X5 had confidence levels below 95% and hence were considered to be insignificant. The rest of variables X1, X2, and X6 having confidence levels above 95% were considered significant and were used in the next optimization using Box-Behnken design. Variables with insignificant effect were not considered for further optimization, but used in all trials at their (-1) level and (+1) level, for the negatively and the positively contributing, respectively. |
| |
| Regression analysis |
| |
| |
| An analysis of variance was performed to evaluate the quadratic model (Equation. (5)). By applying multiple regression analysis on the experimental data, the following second order polynomial equation was found to give the hydrogen yield: |
| |
| Y = 210.7 + 30.8X1 + 15.8X2 + 8.0X3 – 44. 5X1 2 – 37.9X2 2 – 37.7 X3 2 – 17.7X1X2 + 2.5X1X3 + 7.0X2X3 (5) |
| |
| Where Y is the predicted hydrogen yield; X1, X2 and X3 are the coded values of glucose (g/l), initial pH and ferric chloride (mg/l). The regression coefficients were estimated for the model and the corresponding P-values were shown in the Table 4. ANOVA analysis showed that the linear and quadratic effect of glucose, initial pH and ferric chloride, and the interactive effect of glucose and initial pH and initial pH and ferric chloride on hydrogen yield were highly significant (p < 0.05). This indicates that these terms had great impact on hydrogen production and yield. However, the interactive effect between ferric chloride and glucose concentrations on hydrogen yield was not significant (p > 0.05). The Model F-value of 104.6 implies the model is significant (Table 5). There is only a 0.01% chance that a “Model F-Value” this large could occur due to noise. Lack-of-fit F-value is another evidence to confirm the model significant. There is only 18.7% chance that a "Lack of Fit F-value" this large could occur due to noise. These investigations confirm that the Equation. (5) correlated reasonably well with the experimental data and demonstrated well the effect of each independent variable on the hydrogen yield. |
| |
|
|
Table 4: Model coefficients estimated by multiple linear regressions. |
|
| |
|
|
Table 5: ANOVA results of the experimental response at different factor levels. |
|
| |
| Effect of independent variables |
| |
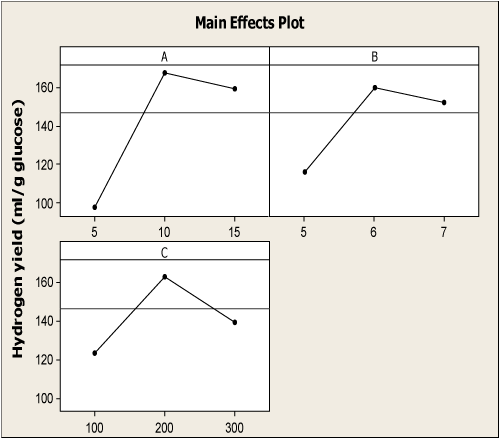
| Figure 1a represented the effect of three factors on the H2 yield. Increasing H2 yields were observed with increasing initial pH from 5.0 to 6.15, and then declined with further increase to 7.0. In many studies with pure cultures, increasing H2 yields have been observed at lower pH values [21,22]. The observations in this study are in agreement with the findings that increasing pH results in a decrease in H2 yield [20,23-25]. It was evident from the Figure 1b, that increased glucose level from 10 g/l to 16.56 g/l had significant effect on H2 yield. Further increase from 16.56 g/l led to decrease in H2 yield. It has already been reported that substrate inhibition gets predominant at higher glucose concentration because this modifies the metabolic pathways [10,26]. An increase in substrate concentration could lead to a partial pressure in the fermentation system. Increased partial pressure level in the headspace of the system will switch the process from acidogenesis to solventogeneis, thus inhibiting the hydrogen production [27]. The generation of hydrogen by fermentative bacteria accompanies the formation of volatile fatty acid as metabolic products. Since alcohol production involves the consumption of hydrogen in the form of reducing equivalents such as NADH, it is inevitable that fermentation conditions that favor the metabolism of sugar to alcohols reduce hydrogen production. Solvent production would cause a drop in the culture pH and subsequent reduction in the hydrogen production [28]. Similarly, in Figure 1c, increasing H2 yields have been observed at increased FeCl3 concentration from 100 mg/l to 213.13 mg/l, and then declines. These results were in agreement with Yang and Shen [29] and Lee et al. [30]. The addition of external iron concentration promoted the bioactivity of hydrogen producing microbe [31]. Iron is the important micronutrient to form Fe-hydrogenase or other enzymes which almost all biohydrogen production needs fundamentally [32]. Fe-hydrogenase is an iron containing enzyme that catalyzes the reversible oxidation of molecular H2 from protons and electrons [33]. All these indicate that H2 yield increases significantly up to the optimal conditions of initial pH, glucose level and iron concentration. |
| |
|
|
Figure 1: (a, b, c) Main effects plot of three experimental factors on H2 yield [A –Glucose (g/l); B – Inoculum (%v/v) and C – Tryptone (g/l)]. |
|
| |
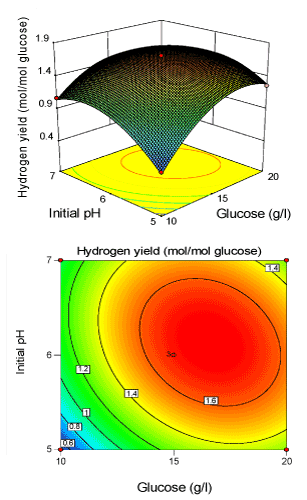
| 3 D response surface plot and 2 D contour plots were constructed using the Design expert 8.0 and were produced in Figures 2-4. here, each contour plot represents the effect of two independent variables taking the third variable at its centre level. The shape of the contour plot explicitly demonstrates the mutual or combined effect of the independent variables on the response variable. It was obvious from the Figures 2-4, the entire response surface plot had a clear peak and their corresponding contour plots had a clear highest point. This confirms that the maximum hydrogen yield was achieved inside the design boundary. As can be seen from Figure 2, the relative effect between initial pH and glucose concentration (X1X2) was highly significant. It means the change in initial pH and glucose level led to the change in hydrogen yield. The inclination angle of the principal axis indicates that the positive effect of increased glucose level on yield was more pronounced as initial pH increased. The 2-D contour plot with respect to glucose and initial pH showed a clear elliptical diagonally on plot, suggesting that glucose and initial pH were interdependent. |
| |
|
|
Figure 2: 3-D surface plot and 2-D contour plot on H2 yield (Initial pH; Glucose). |
|
| |
|
|
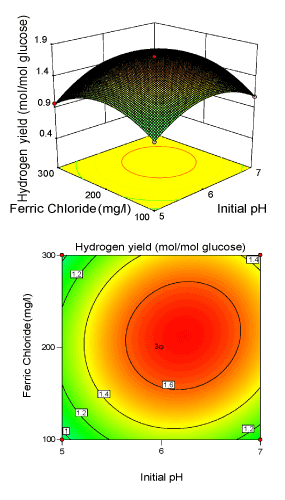
Figure 3: 3-D surface plot and 2-D contour plot on H2 yield (Ferric chloride; Initial pH). |
|
| |
|
|
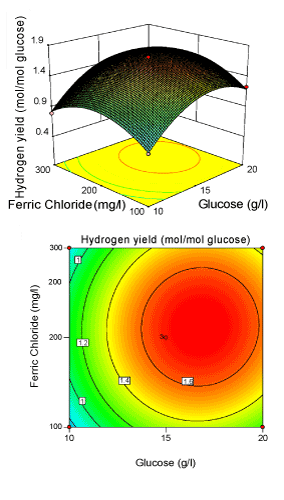
Figure 4: 3-D surface plot and 2-D contour plot on H2 yield (Glucose; Ferric chloride). |
|
| |
| Figure 3 illustrated the effect of initial pH (X2) and FeCl3 (X3) concentration for the hydrogen production with glucose concentration (X1) kept constant. Hydrogen yield increased with increasing FeCl3 and initial pH to optimum conditions, and then decreased with a further increase. It was obvious that yield of Enterobacter aerogenes was sensitive, when FeCl3 concentration was subjected to small alteration above 213.13 mg/l. The model showed that the interactive effect of FeCl3 and initial pH was most significant with p-value<0.05, indicating that this effect has great impact on H2 yield. Presence of Fe3+ in the fermentative medium would facilitate the iron supply for survival of bacteria [30,34-37]. The elliptical nature of the contour plots indicates that the mutual interactions between the two independent variables (X2, X3) are significant. These significant interaction effects mean that the effect of initial pH on yield is dependent on the level of Fe3+ used. Figure 4 illustrated the effects of glucose level (X1) and FeCl3 (X3) level on biohydrogen production with initial pH (X2) at the centre level. The response H2 yield showed a peak at 16.56 g/l of glucose and 213.13 mg/l of FeCl3. The angle of inclination of the principle axis was slight in Figure 4 explaining that the hydrogen yield was nearly less dependent than the other two interactive effect (Figure 2 and 3). |
| |
| Model verification and validation |
| |
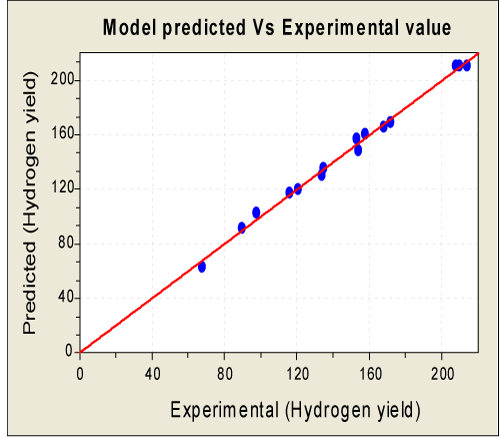
| The optimal factor setting was identified by the D-optimality analysis and their values in the actual were: glucose - 16.56 g/l, initial pH – 6.15 and ferric chloride – 213.13 mg/l respectively. At these optimal conditions, the maximum predicted value of hydrogen yield calculated was 1.74 mol H2/mol glucose. In order to confirm the predicted results of the model, an experiment in triplicate was carried out at an optimal condition and was found that a maximum hydrogen yield of 1.69 mol H2/mol glucose was obtained. The experimental response (1.69 mol H2/mol glucose) was approximately 2.8% lesser than the predicted maximum response. From the Equation (5), computed response evaluated are correlated reasonably well with the experimental values with the coefficient of determination (R2) 0.9967 (Figure 5). |
| |
|
|
Figure 5: Plot for model predicted and the experimental values for hydrogen yield [Hydrogen yield is in ml H2/g glucose). |
|
| |
| Cumulative hydrogen production by modified Gompertz model |
| |
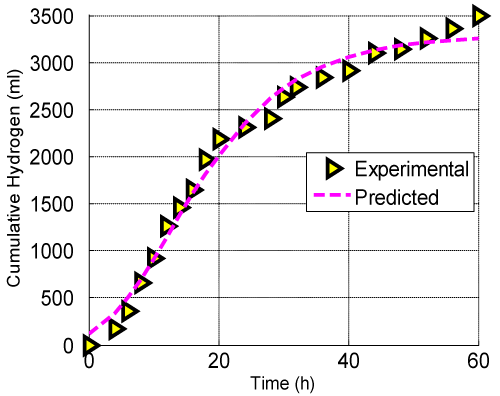
| Cumulative hydrogen produced from glucose was plotted in Figure 6. This curve was fitted by using modified Gompertz equation at an optimal condition obtained by the D-optimality analysis. The values of P, Rm and λ were evaluated by best fitting the cumulative hydrogen production data in the Equation (4) using the Matlab program (Table 6). R2 value of 0.9876 indicated a strong correlation between the experimental data and the fit. Table 7 illustrated the comparison of hydrogen yield of the present study with the other cited in literatures. The results obtained in this study were higher than that reported by Tanisho et al. [38]. Moreover, the results were in good agreement with Lin et al. [39]. |
| |
|
|
Figure 6: Effect of time on cumulative hydrogen production at the optimal conditions (glucose=16.56g/l; initial pH=6.15 and Fecl3=213.13mg/l). |
|
| |
|
|
Table 6: Modified Gompertz model kinetic parameters for hydrogen production at an optimal condition. |
|
| |
|
|
Table 7: Comparison of biohydrogen production obtained in this study with other reports cited in literature. |
|
| |
| Conclusions |
| |
| The response surface design was employed for the optimization of hydrogen yield from glucose by Enterobacter aerogenes. Plackett– Burman design and Box–Behnken design were applied to screen the significant process variables and to identify the optimal values for the maximum hydrogen production. The R2 value of 0.9947 confirms the accuracy of model fitness with the experimental data. The linear, quadratic and interactive effects of glucose, initial pH and ferric chloride had been explained significant influence on biohydrogen production. Maximum H2 yield of 1.69 mol H2/mol glucose was achieved under the optimal factor setting of three factors using Enterobacter aerogenes. Based on the experimental conditions, the response model can accurately predict the H2 yield and therefore the model is said to be valid over the factor space under consideration. The above results explicitly indicate that the statistical design methodology could be able to offer an efficient and feasible approach for hydrogen production optimization. |
| |
| |
| References |
| |
- Nath K, Das D (2004) Improvement of fermentative hydrogen production: Various approaches. Appl Microbiol Biotechnol 65: 520-529.
- Kapdan IK, Kargi F (2006) Biohydrogen production from waste materials. Enzyme Microb Technol 38: 569-582.
- Levin D, Pitt L, Love M (2004) Biohydrogen production: Prospects and limitations to practical application. Int J Hydrogen Energy 29: 173-185.
- Kumar P, Satyanarayana T (2007) Optimization of culture variables for improving glucoamylase production by alginate-entrapped Thermomucorindicae-seudaticae using statistical methods. Bioresour Technol 98: 1252-1259.
- Wang YX, Lu ZX (2005) Optimization of processing parameters for the mycelial growth and extracellular polysaccharide production by Boletussp. ACCC 50328. Proc Biochem 40: 1043-1051.
- Annadurai G, Balan SM, Murugesan T (1999) Box–Behnken design in the development of optimized complex medium for phenol degradation using Pseudomonas putida (NICM 2174). Bioprocess Eng 21: 415-421.
- Lee KS, Hsu YF, Lo YC, Lin PJ, Lin CY, et al. (2008) Exploring optimal environmental factors for fermentative hydrogen production from starch using mixed anaerobic microflora. Int J Hydrogen Energy 33: 1565-1572.
- Pan CM, Fan YT, XingY, Hou HW, Zhang M (2008) Statistical optimization of process parameters on biohydrogen production from glucose by Clostridium sp.Fanp2. Bioresour Technol 99: 3146-3154.
- Mu Y, Wang G, Yu HQ (2006) Response surface methodological analysis on biohydrogen production by enriched anaerobic cultures. Enzyme Microb Technol 38: 905-913.
- Fabiano B, Perego P (2002) Thermodynamic study and optimization of hydrogen production by Enterobacter aerogenes. Int J Hydrogen Energy 27: 149-156.
- Kurokawa T, Tanisho S (2005) Effects of formate on fermentative hydrogen production by Enterobacter aerogenes. Mar Biotechnol 7: 112–118.
- Nakashimada Y, Rachman MA, Kakizono T, Nishio N (2002) Hydrogen production of Enterobacter aerogenes altered by extracellular and intacellular redox states. Int J Hydrogen Energy 27: 1399–1405.
- Kim IS, Hwang MH, Jang NJ, Hyun SH, Lee ST (2004) Effect of low pH on the activity of hydrogen utilizing methanogens in biohydrogen process. Int J Hydrogen Energy 29: 1133-1140.
- Kim SH, Han SK, Shin HS (2006) Effect of substrate concentration on hydrogen production and 16S rDNA-based analysis of the microbial community in a continuous fermenter. Proc Biochem41: 199-207.
- Taguchi F, Mizukami N, Hasegawa K, Saito-Taki T (1994) Microbial conversion of arabinose and xylose to hydrogen by a newly isolated Clostridium sp. no.2. Can J Microbiol 40: 228-233.
- Venkatamohan S, Lalit Babu V, Sarma PN (2008) Effect of various pretreatment methods on anaerobic mixed microflora to enhance biohydrogen production using dairy wastewater as a substrate. Bioresour Technol 99: 59-67.
- Plackett RL, Burman JP (1946) The design of optimum multi-factorial experiments. Biometrika 33: 305- 325.
- Box GEP, Behnken EW (1960) Some new three level designs for the study of quantitative variables. Technometrics 2: 455-475.
- Nath K, Kumar A, Das D (2005) Hydrogen production by Rhodobacter sphaeroides strain O.U 001 using spent media of Enterobacter cloacae DM11. Appl Microbiol Biotechnol 68: 533-541.
- Zhang T, Liu H, Fang HHP (2003) Biohydrogen production from starch in wastewater under thermophilic condition. J Environ Manage 69: 149-156.
- Kataoka N, Miay A, Kiriyama K (1997) Studies on H2 production by continuous culture system of hydrogen producing anaerobic bacteria. Water Sci Technol 36: 41-47.
- Kumar N, Das D (2000) Enhancement of hydrogen production by Enterobacter cloacae IIT BT 08. Proc Biochem 35: 589-593.
- Lee YJ, Miyahara T, Noike T (2002) Effect of pH on microbial hydrogen fermentation. J Chem Technol 77: 694-698.
- Fang HHP, Liu H (2002) Effect of pH on hydrogen production from glucose by a mixed culture. Bioresour Technol 82: 87-93.
- Evvyernie D, Yamazaki S, Morimoto K, Karita S, Kimura T, et al. (2000) Identification and characterization of Clostridium paraputrificum M-21, a chitinolytic, mesophilic and hydrogen-producing bacterium. J Biosci Bioeng 89: 596-601.
- Oh Y-K, Seol E-H, Kim JR, Park S (2003) Fermentative biohydrogen production by a new chemo-heterotrophic bacterium Citrobacter sp.Y19. Int J Hydrogen Energy 28: 1353-1359.
- Fan YT, Li CL, Lay JJ, Hou HW, Zhang GS (2004) Optimization of initial substrate and pH levels for germination of sporing hydrogen-producing anaerobes in cow dung compost. Bioresour Technol 91: 189-193.
- Das D, Veziroglu TN (2001) Hydrogen production by biological processes: A survey of literature. Int J Hydrogen Energy 26: 13-28.
- Yang H, J Shen (2006) Effect of ferrous iron concentration on anaerobic biohydrogen production from soluble starch. Int J Hydrogen Energy 31: 2137-2146.
- Lee YJ, T Miyahara, T Noike (2001) Effect of iron concentration on hydrogen fermentation. Bioresour Technol 80: 227-331.
- Zhang Y, Liu G, Shen J (2005) Hydrogen production in batch culture of mixed bacteria with sucrose under different iron concentrations. Int J Hydrogen Energy 30: 855-860.
- Junelles AM, Janati Idrissi R, Petitdemange H, Gay R (1988) Iron effect on acetone –butanol fermentation. Curr Microbiol 17: 299-303.
- Nicolet Y, Cavazza C, Fontecilla-Camps JC (2002) Fe-only hydrogenases: structure, function and evolution. J Inorg Biochem 91: 1-8.
- Li CL, Fang HHP (2007) Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Cri Rev Environ Sci Technol 37: 1-3
- Chong ML, Rahman NAA, Yee PL, Aziz SA, Rahim RA, et al. (2009) Effects of pH, glucose and iron sulfate concentration on the yield of biohydrogen by Clostridium butyricum EB6. Int J Hydrogen Energy 34: 8859-8865.
- Guo WQ, Ren NQ, Wang XJ, Xiang WS, Ding J, et al. (2009) Optimization of culture conditions for hydrogen production by Ethanoligenens harbinense B49 using response surface methodology. Bioresour Technol 100: 1192-1196
- Long C, Cui J, Liu Z, Liu Y, Long M, et al. (2010) Statistical optimization of fermentative hydrogen production from xylose by newly isolated Enterobacter sp. CN1. Int J Hydrogen Energy 35: 6657-6664.
- Tanisho S, Kamiya N, Wakao N (1989) Hydrogen evolution of Enterobacter aerogenes depending on culture pH: mechanism of hydrogen evolution from NADH by means of membrane-bound hydrogenase. Biochim Biophy Acta 973: 1-6.
- Lin PY, Whang LM, Wu YR, Ren WJ, Hsiao CJ, et al. (2007) Biological hydrogen production of the genus Clostridium: metabolic study and mathematical model simulation. Int J Hydrogen Energy 32: 1728-1735.
|
| |
| |