|
|
| Noer Abyor Handayania, Dessy Ariyantib and Hadiyanto* |
| Chemical Engineering Department, Engineering Faculty, Diponegoro University, Jl. Prof. Sudharto, Tembalang, Semarang 50239, Indonesia |
| *Corresponding authors: |
Hadiyanto
Chemical Engineering Department
Engineering Faculty, Diponegoro University
Tembalang, Semarang 50239, Indonesia
Tel: +62- 813-26477628
Fax: +62-024-7460058
E-mail: h.hadiyanto@undip.ac.id |
|
| |
| Received November 22, 2011; Published July 30, 2012 |
| |
| Citation: Handayania NA, Ariyantib D, Hadiyanto (2012) Potential Production of Polyunsaturated Fatty Acids from Microalgae. 1: 180. doi:10.4172/scientificreports. 180 |
| |
| Copyright: © 2012 Handayania NA et al., This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Currently, public awareness of healthcare importance increase. Polyunsaturated fatty acid is an essential nutrition for us, such arachidonic acid, docosahexaenoic acid and eicosapentaenoic acid. The need of polyunsaturated fatty acid generally derived from fish oil, but fish oil has a high risk chemical contamination. Microalgae are single cell microorganism, one of Phaeodactylum tricornutum which have relatively high content of eicosapentaenoic acid (29,8%). Biotechnology market of Polyunsaturated fatty acid is very promising for both foods and feeds, because the availability of abundant raw materials and suitable to develop in the tropics. This literature review discusses about the content of Polyunsaturated fatty acid in microalgae, omega-3, omega-6, Polyunsaturated fatty acid production processes, and applications in public health. |
| |
| Keywords |
| |
| Poly-unsaturated fatty acid; PUFA; Microalgae; Health |
| |
| Introduction |
| |
| Polyunsaturated fatty acid (PUFA) is an essential component which cannot be synthesized by human. Therefore, PUFA must be provided through the diet [1]. Omega-3 (PUFA n-3) and omega-6 (PUFA n-6) are well known derivative products of PUFA. |
| |
| PUFAs are found in animals, plants, fungi, and microalgae. Nowadays, food habits in society are characterized by a high consumption of fast food which contain low portion of PUFAs. Some authors have compared the composition of fast food with Japanese food [2,3] and Mediterranean food [2,4] that mostly source in fish. These authors concluded that then-6:n-3 ratio in blood, in people who consume Japanese and Mediterranean food, is close to 2:1; while people who consume fast food, it can reach up to 25:1, so far than desired. For these reason, nutritionists educate the need to consume fish and green vegetables to reduce the risk of heart diseases [2]. In contrast, the availability of fish is highly dependent on season may hamper the continuity of food [5] thus encouraging the discovery of alternative sources of PUFA. Basically, fish do not produce PUFA; they will get it by eating microalgae. This realization has turned microalgae into one of the most important producers of PUFA [6,7,8,5,1]. |
| |
| Exploration of microalgae is not used as food diversification effort only, but also intended to empower agricultural land which is not feasible. As a tropical country, Indonesia has suitable temperature and contains high level of salt so it is feasible for the growth of microalgae [6]. This literature review discusses about the content of PUFA in microalgae, PUFA production processes, and applications in public health. |
| |
| Polyunsaturated fatty acid (PUFA) |
| |
| Microalgae are autotrophic organisms which grow rapidly through photosynthesis like land based plants. Their unicellular structure allows them to convert solar energy into chemical energy easily [9]. Microalgae can grow to high densities population by using only light, carbon dioxide, water, and other inorganic compounds [10]. |
| |
| Microalgae have a composition of carbohydrate, protein, and fats are widely used to meet food needs and public’s health. Nutritional supplement products from microalgae can be found in form of powders, tablets, capsules, and concentrates (Table 1). The average composition of proteins, fats, and carbohydrates in microalgae are 12-35%; 7,2-23%; 4,6-23% (%dry weight) [11]. Different nutrition, environment, and growth phases can influence the nutritional composition in microalgae, including fatty acid composition [12,11]. |
| |
|
|
Table 1: Commercial food products from microalgae (Gouveia, L., et al., 2008) |
|
| |
| Fatty acids are organic compounds formed by long chain hydrocarbon and carboxylic group [1]. Based on their nature hydrocarbon chain, fatty acids can be classified into saturated and unsaturated. Polyunsaturated Fatty Acids (PUFA) are a long chain of unsaturated fatty acid. PUFA composition in the Cod liver oil and some microalgae are shown in (Table 2). |
| |
|
|
Table 2: PUFA composition in 320 the Cod liver oil and some microalgae [21] |
|
| |
| Omega-3 fatty acids (PUFA n-3) are unsaturated 74 fatty acids and contained in food as α-linolenic acid (ALA, C18:3, n-3) [13,5]. ALA is the shortest chain of n-3 and mainly found in vegetables oil and nuts [13,5]. Eicosapentaenoic acid (EPA, C20:5, n-3) and docosahexaenoic acid (DHA, C22:6, n-3) are derivatives product from n-3 found in fish and many other microorganisms, such as microalgae and bacteria [8,5,1]. ALA can be converted into EPA and DHA in the body, but the conversion is very limited and inefficient [5], therefore -3 must be provided in the form of dietary supplement. |
| |
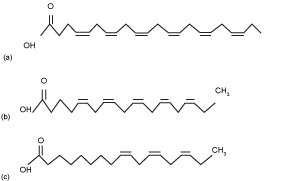
| EPA and DHA have positive effects on health. Recently, EPA proven to prevent coronary heart disease, and lowers blood cholesterol [14]. DHA also has an important role in the development of central nervous system of infants [14]. EPA is widely found in Porphyridium cruentum, and Monodus subterraneus [1], while the best source of DHA derived from microalgae with Schyzochytrium genus [1]. Currently, some government agencies and nutritional organizations recommended daily intake level of DHA and EPA ranging from 0,2-0,3 g/day for the general population [5,15] up to 1,0-4,0 g/day for patient with coronary heart disease [5]. Chemical structure of ALA, EPA, and DHA are shown in (Figure 1). |
| |
|
|
Figure1: (a) DHA, (b) EPA, (c) ALA (McManus, A., et al., 2011) |
|
| |
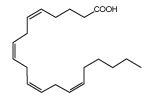
| Omega-6 fatty acid (PUFA n-6) are polyunsaturated fatty 96 acid contained in food as linoleic acid (LA, C18:2, n-6) [5,16]. LA is the shortest chain from n-6 and arachidonic acid (AA, C20:4, n-6) is one of derivatives product from n-6. AA is almost excluded from the water alga and most marine species. The only microalgae which produce AA in significant quantitative is the unicellular rhodophyte: P. cruentum [7]. Other Rhodophytes such as Gracilaria sp has a composition of AA upto 60% of total fatty acids, but the dry weight content doesn’t exceed 0,2% [7]. AA is a biogenetic precursor of the biologically active prostaglandin and leucotrienes with important role in circulatory and central nervous systems [14]. AA is necessary for visual acuity, and it is valuable as an ingredient in formulations of artificial baby food [14]. Chemical structure of AA is shown in (Figure 2). |
| |
|
|
Figure 2: AA (Kiefer, J., et al., 2010) |
|
| |
| Production of PUFA |
| |
| Microalgae production consists of five important stages as shown in (Figure 3). |
| |
|
|
Figure 3: Microalgae production processes. |
|
| |
| Cultivation condition of microalgae: Most microalgae use light and carbon dioxide (CO2) as an energy and carbon sources (photoautotrophic organism) [17,18]. Temperature must remain generally 15-30°C for optimum growth. Growth medium must provide inorgan 118 ic elements that function in cell formation, such as nitrogen, phosphor,and iron [5,17]. |
| |
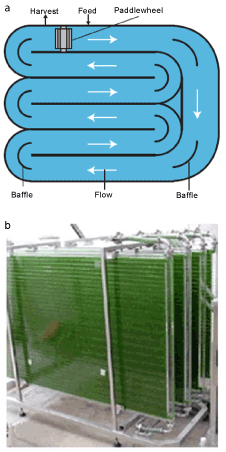
| Cultivation technique of microalgae: Nowadays, there are two most commonly used techniques to cultivate microalgae. These are open race way ponds and photobioreactors [9]. Open ponds are the oldest and simplest systems for cultivation [17]. Enclosed photobioreactors have been used to overcome the contamination and evaporation problems encountered in open ponds [18]. These systems are made of transparent materials and commonly placed outdoors for illumination by natural lights [9]. Photobioreactors have a large surface area to volume ratio [17,18]. Microalgae productivity of photobioreactors can reach 13 times more than open ponds [17,18]. Figure 4 shows the open raceway ponds and photobioreactors systems. |
| |
|
|
Figure 4: (a) open raceway pond and (b) photobioreactor (Wen, Z., at al., 2009). |
|
| |
| Dewatering of microalgae: Flocculation, centrifugation, and filtration are commonly techniques for dewatering microalgae [6,19,9]. Flocculation can be used as initial step that will enhance the ease of next processing. Microalgae have a negative charge, thus they need cationic chemical flocculants for coagulating biomass, such as Al2(SO4)3, FeCl3, dan Fe2(SO4)3 [19,9]. Filtration has proved to be the most competitive technique [9]. The types of filtration that can be used are dead end filtration, microfiltration, ultrafiltration, pressure filtration, and tangential flow filtration [9]. |
| |
| Disruption: Is the separation process of lipids and other non lipids chain [20]. This process is necessary before extraction stage. Disruption agents must not degrade the lipids [14]. There are five methods of disruption that can be used, (i) autoclave (125°C, 1,5 MPa), (ii) beadbeater (bead diameter 0,1 mm, speed 2800 rpm), (iii) microwave (100°142 C, 2450 MHz), (iv) sonication using sonicator (resonance 10 KHz for 5min), and (v) osmotic shock processes [21]. |
| |
| Extraction of microalgae: Can be classified into two methods, (1) mechanical methods (Expression/Expeller press and Ultrasonicassisted extraction) and chemical methods (Hexane Solvent Method, Soxhlet extraction, and Supercritical fluid Extraction) [12,11]. Each of these methods has drawbacks: (a) the mechanical methods requires drying process which is energy intensive, (b) the use of chemical solvent should be considered to the level of safety and health, (c) supercritical extraction requires a high pressure equipment that is expensive and energy intensive [22]. |
| |
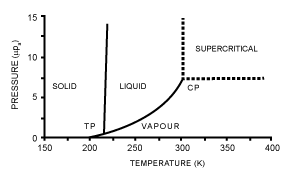
| Supercritical Fluid Extraction (SFE): (Figure 5) shows pressuretemperature phase diagram for carbon dioxide. In this diagram, the vapor-liquid equilibrium curve starts at the triple point (TP), where both of three phases, solid, liquid, and vapor are in equilibrium. This curves ends at the critical point (CP), where the meniscus separating vapor and liquid phases disappears and only a single phases formed. Carbon dioxide (CO2) is in the supercritical state if its temperature and pressure higher than its critical temperature (304,1K) and pressure (7,38MPa). In its critical region, CO2 is sensitive with very small changes in temperature and pressure [23]. Supercritical CO2 has been the most used supercritical fluid, because non-flammable, non toxic, inexpensive, and relatively inert [23]. On the other hand, the adding of entrainer, a substance which has a volatility intermediate between the compound to be extracted and the supercritical fluid, can increase the solvent power of CO2 [23]. Due to its non polar behavior, CO 167 2 cannot be used to extract the polar compound. However, the addition of polar entrainer such as water, ethanol, and methanol, thus polar compound extraction can be done [23]. |
| |
|
|
Figure 5: Pressure – temperature phase diagram for carbon dioxide (CO2) (Mendes, R.L., et 270 al., 2003). |
|
| |
| Conclusion |
| |
| PUFA are polyunsaturated fatty acid that has positive effects on health such as lowering cholesterol level and the risk of heart disease. Due to their continuity of their raw materials, easily developed in the tropics, and not susceptible to chemical contamination, microalgae are an alternative source of PUFA which is potential to be developed in Indonesia. PUFA extraction from microalgae can be done by several methods, mechanical and chemical methods. The potency of microalgae as feedstock PUFA is very large, it is necessary to study deeper about the extraction and purification of PUFA which is more economic with good quality and quantity. |
| |
| |
| References |
| |
- Ward OP, Singh A, (2005) Omega-3/6 fatty acids: Alternative sources of production. Process Biochemistry 40: 3627-3652.
- Rubio-Rondriguez N, Beltran S, Jaime I, De Diego S, Sanz MT, et al. (2010) Production of omega-3 polyunsaturated fatty acid concentrates: A review. Innovative Food Science and Emerging Technologies 11: 1-12.
- Kamei M, Ki M, Kawagoshi M, Kawai N (2002) Nutritional Evaluation of Japanese Take-out Lunches Compared with Western-style Fast Foods Supplied in Japan. Journal Of Food Composition and Analysis 15: 35-45.
- Thellen JJ, Ohlrogge JB (2002) Metabolic Engineering of Fatty Acid Biosynthesis in 251 Plants. Metabolic Engineering 4: 12-21.
- Kalogeropoulos 215 N, Chiou A, Gavala E, Christea M, Andrilkopoulos NK (2010) Nutritional evaluation and bioactive microconstituents (carotenoids, tocopherols, sterols and squalene) of raw and roasted chicken fed on DHA-rich microalgae. Food Research International 43: 2006-2013.
- Agustini NWS, Kabinawa INK Pengaruh Konsentrasi nitrat Sebagai Sumber Nitrogen dalam media Kultur Terhadap Pembentukan Asam Arakidonat dari Mikroalga Phorphyridium cruentum. Proses Kimia Ramah Lingkungan ISSN 1410-9891
- Bigogno C, Khozin-Goldberg I, Boussiba S, Vonshak A, Cohen Z (2002) Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 60: 497-503.
- Guedes AC, Amaro HM, Barbosa CR, Pereira RD, Malcata FX (2011) Fatty Acid Composition of Several Wild Microalgae and Cyanobacteria, with a focus on Eicosapentaenoic, Docosahexaenoic and Linolenic Acids for Eventual Dietary Uses. Food Research International 44: 2721-2729.
- Harun R, Singh M, Forde GM, Danquah MK (2010) Bioprocess engineering of microalgae to produce a variety of consumer products. Renewable and Sustainable Energy Reviews 14: 1037–1047.
- John RP, Anisha GS, Nampoothiri KM, Pandey A (2011) Micro and macroalgal biomass: A renewable source for bioethanol. Bioresource Technology 102: 186-193.
- Anonymous (2011) Nutritional value of micro-algae. www.fao.org , Akses: 24 Juni 2011, Pukul 10.00 WIB
- Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and nother applications: A review. Renewable and Sustainable Energy Reviews 14: 217–232.
- Chew YL, Lim YY, Omar M, Khoo KS (2008) Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT 41: 1067-1072.
- Medina AR, Cerda´n LE, Gime´nez AG, Paez BC, Gonzalez MJI, et al. (1999) Lipase-catalyzed esterification of glycerol and polyunsaturated fatty acids from fish and microalgae oils. Journal of Biotechnology 70: 379-391.
- Ruxton CHS, Reed SC, Simpson MJA, Millington KJ (2004) The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet 70: 449-459.
- McCusker MM (2010) Healing fats of the skin: the structural and immunologic roles of the ?-6 and ?-3 fatty acids. Clin Dermatol 28: 440-451.
- Chisti J (2007) Biodiesel from microalgae. Biotechnology Advances 25: 294-306.
- Wen Z, Johnson MB (2009) Microalgae as a Feedstock for Biofuel Production. Virginia Cooperative Extension 442-886.
- Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. Journal of Biotechnology 70: 313-321.
- Medina AR, Grima EM, Gimenez AG, Gonzalez MJI (1997) Downstream Processing Of Algal Polyunsaturated Fatty Acids. Biotechnol Adv 16: 517-580.
- Lee JY, Yoo C, Ahn CY, Oh HM (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresource Technology 101: S75-S77.
- Mendes RL, Nobre BP, Cardoso MT, Pereira AP, Palavra AF (2003) Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorganica Chimica Acta 356: 328-334.
- Anonymous, (2011), Algae Oil Extraction., www.oilgae.com, Akses: 21 Juni 2011, Pukul 14.00 WIB 10
- Kiefer J, Noack K, Bartelmess J, Walter C, Dörnenburg H, et al. (2010) Vibrational structure of the polyunsaturated fatty acids eicosapentaenoic acid and arachidonic acid studied by infrared spectroscopy. Journal of Molecular Structure 965: 121-124.
- McManus A, Merga M, Newton W (2011) Omega-3fatty acids.What consumers need to know. Appetite 57: 80-83.
- Gouveia L, Batista AP, Sousa I, Raymundo A, dan Bandarra NM (2008) Microalgaein Novel Food Products. Food Chemistry Research Developments 75-112.
- Jiang Y, Chen F, Liang S (1999) Production potential of docosahexaenoic acid by the heterotrophic marine dinoflagellate Crypthecodinium cohnii. Process Biochemistry 34: 633-637.
|
| |
| |