| Research Article |
Open Access |
|
| Mittal Deepak Kumar*, Joshi Deepmala and Shukla Sangeeta |
| Reproductive Biology and Toxicology Laboratory, School of Studies in Zoology, Jiwaji University, Gwalior, India |
| *Corresponding authors: |
Mittal Deepak Kumar
Reproductive Biology and Toxicology Laboratory
School of Studies in Zoology, Jiwaji University
8-Samta marg panchseel nagar mela ground
thatipur, Gwalior-474011(M.P.), India
Tel: +91-9425771967, 07415401930
E-mail: deepakmittal05@gmail.com |
|
| |
| Received April 02, 2011; Published August 13, 2012 |
| |
| Citation:Kumar MD, Deepmala J, Sangeeta S (2012) Hepatoprotective Effects of Polygonum Bistorta and Its Active Principles on Albino Rats Intoxicated with Carbon Tetrachloride and Paracetamol. 1: 226. doi:10.4172/scientificreports.226 |
| |
| Copyright: © 2012 Kumar MD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Herbal preparations have been recommended in alternative systems of medicine for the treatment of hepatic disorders. Among the various studies involved in hepatotoxic effect of CCl4 (1.5 ml/kg, i.p.) and paracetamol (2 g/kg, p.o.) is oxidative damage through free radical generation. Drug-induced hepatotoxicity is still a significant unresolved clinical problem as liver is the most common site of damage. Polygonum Bistorta (PB) is powerful astringent, demulcent, diuretic, febrifuge, laxative, strongly styptic and rich in tannins. No systematic study has been done on protective efficacy of (PB) and its active principles Tannic acid (TA) and Resveratrol to treat hepatic diseases. The goal of the present work is to evaluate and compare the efficacy of root extract of PB (100 mg/kg), TA (25 mg/kg, p.o.) and Resveratrol (30 mg/kg, p.o.) against toxicants induced damage in liver and kidney. The activities of transaminases, alkaline phosphatase and protein were increased in serum after 48 h days of toxicants administration. A significant rise was observed in lipid peroxidation level however reduced glutathione content was observed. A concomitant fall was observed in the enzymatic activities of adenosine triphosphatase, glucose-6- phosphatase. Administration of PB and TA and Resveratrol significantly brought the values of studied parameters towards normal and also reversed the histopathological alterations in liver and kidney. Thus, it may be concluded that PB and TA can be used to reduce the hepatorenal damage and may serve as an alternative medicine. Thus it may be concluded that TA (25 mg/kg) were found more effective values towards control. |
| |
| Keywords |
| |
| CCl4; Paracetamol; Polygonum bistorta; Tannic acid; Serum transaminases; Comet assay |
| |
| Introduction |
| |
| Herbal medicines derived from plant extracts are being increasingly utilized to treat a wide variety of clinical diseases, through relatively little knowledge about their mode of action is available. There is a growing interest in the pharmacological evaluation of various plants used in Indian traditional system of medicine. |
| |
| Liver disease is still a worldwide health problem. Unfortunately, conventional or synthetic drugs used in the treatment of liver diseases are inadequate and sometimes can have serious side effects. In the absence of a reliable liver protective drug in modern medicine there are a number of medicinal preparations in Ayurveda recommended for the treatment of liver disorders [1]. The World Health Organization (WHO) estimates that 80% of the population of developing countries relies on traditional medicines, for their primary health care needs. Also, modern pharmacopoeia still contains at least 25% drugs derived from plants and many others which are synthetic analogues built on prototype compounds isolated from plants [2]. |
| |
| The plant Polygonum bistorta Linn. (Family: Polygonacae) is used in the Siddha system of medicine. This plant possesses a broad spectrum of antibiotic, antibacterial, and anticancer activity. The stem bark of the plant is an astringent and is used in the treatment of headache, fever, skin diseases, tumors, diseases of the blood, dysentery and diarrhoea. Preliminary pharmacological studies of the plant revealed that the aqueous extract of roots of P. bistorta shows anti-inflammatory, antiulcer and antimicrobial activity. The fresh roots of the plant have anti cancerous activity [3]. The roots contain up to 21% tannin. These are astringent, bitter plant polyphenols that either bind and precipitate or shrink proteins [4]. According to Rashid et al. (1985) [5], "Tannic acid is the commercial term for a mixture of large gallotannins, trigallic, m-digallic, and gallic acid, which is extracted from plant material. Resveratrol (trans-3, 4’, 5-trihydroxystilbene) occurs naturally in grapes and a variety of medicinal plants. In plants, resveratrol functions as a phytoalexin that protects against fungal infection [6]. |
| |
| An attempt has been made to compare the hepatoprotective potential of extract with its active principle using battery of liver function tests. |
| |
| Materials and Methods |
| |
| Plant material |
| |
| The plant material was collected by the authorized ayurvedic dealer and was identified by the Botany Department of Jiwaji University, Gwalior. |
| |
| Preparation of plant extract |
| |
| Root material of plant was shade, dried, coarsely powdered and allowed to heat in distill water at high temperature of water bath. The inorganic material was precipitated and filtered off. The filtrate was again concentrated in a china dish and dried in vacuum. The yield of the extract was (10.4%) w/w of powdered aqueous extract, which was stored in refrigerator for further use. The aqueous extract was evaporated and dried in vacuum. |
| |
| Chemicals |
| |
| All chemicals used in this study were of analytical grade and obtained from Sigma-Aldrich, E-Merck and Loba Chemicals Pvt. Ltd. All diagnostic kits used in the experiments were procured from E-Merck. |
| |
| Experimental animals |
| |
| Studies were carried out by using adult albino male rats of Sprague Dawley strain (150 ± 10 g) were used for the present investigation. Animals were housed under standard conditions (25 ± 2 °C, 60-70% relative humidity and 14 h light and 10 h dark). The rats were fed on standard pellet diet (Pranav Agro Industries, New Delhi) and water ad libitum. Animals were treated and cared for in accordance with the guidelines recommended by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Govt. of India, Ministry of Environment & Forests (Animals Welfare Division), Chennai. |
| |
| The rats were randomly allocated to six animals in each group and treated as below. |
| |
| Experimental design |
| |
| The animals were administered paracetamol at a dose of (2 g/kg, p.o.) and CCl4 at a dose of (1.5 ml/kg, i.p.) followed by therapeutic agents at effective doses for 24 h and silymarin was given as positive control. The whole set of experiment was divided into six groups six animals each. |
| |
| Experiment No. 1: Acute study |
| |
| • Group 1: Normal (vehicle only) |
| |
| • Group 2: Experimental control (CCl4 1.5 ml/kg, i.p. once only) |
| |
| • Group 3: CCl4 + PB extract (100 mg/kg, p.o) |
| |
| • Group 4: CCl4 + Tannic acid (25 mg/kg, p.o.) |
| |
| • Group 5: CCl4 + Resveratrol (30 mg/kg p.o.) |
| |
| • Group 6: CCl4 + Silymarin (50 mg/kg, p.o.) |
| |
| Experiment No. 2: Acute study |
| |
| • Group 1: Normal (vehicle only) |
| |
| • Group 2: Experimental control (APAP 2/kg, p.o., once only) |
| |
| • Group 3: APAP + PB extract (100 mg/kg, p.o.,) |
| |
| • Group 4: APAP + Tannic acid (25 mg/kg, p.o.,) |
| |
| • Group 5: APAP + Silymarin (50 mg/kg, p.o.,) |
| |
| Experiment No. 3: Determination of cell viability by MTT assay. |
| |
| • Group 1: Medium (100 μl/L) |
| |
| • Group 2: CCl4 (10 mM) |
| |
| • Group 2-4: PB extract (10, 30 and 100 μl/L) |
| |
| • Group 5-7: Tannic acid (10, 30 and 100 μl/L) |
| |
| Biochemical assays |
| |
| Blood samples of rats were withdrawn by puncturing the retroorbital venous sinus [7] and serum was processed for the estimation of blood and tissue parameters. Albumin, bilirubin, urea and creatinine concentration in serum was measured by diagnostic kits. |
| |
| Histopathological assays |
| |
| Liver and kidney were fixed in Bouin’s fluid for light microscopic studies. |
| |
| Statistical analysis |
| |
| The data were expressed as mean value ± S.E. statistical significance of difference between various treatments were analyzed by Student’s‘t’ test followed by one-way analysis of variance (ANOVA) according to Snedecor and Cochran (1994) [8]. P values ≤ 0.05 were considered as statistically significant. |
| |
| Results and Observations |
| |
| Graph 1 (a-f) assesses the effect of CCl4 followed by various therapeutic agents on liver function tests. Administration of CCl4 caused significant elevation in albumin, bilirubin, triglyceride, urea, creatinine, and cholesterol concentration in serum (P ≤ 0.05). Therapy of PB and tannic acid decreased serum contents significantly (P ≤ 0.05) [9]. All contents of serum were significantly recovered by all the treatment groups i.e. PB, tannic acid and resveratrol (P ≤ 0.05). PB and TA showed significant changes when compared with resveratrol at 5 % level by Tukey’s HSD post hoc test. Comparison between TA and PB clearly showed that TA was significantly effective (P ≤ 0.05). |
| |
| Acid and alkaline phosphatase |
| |
| Single bolus dose of CCl4 caused significant rise (P ≤ 0.05) in the enzymatic activity of acid phosphatase in liver and kidney (Graph 2 a-b). Treatment of PB and TA along with CCl4 afforded significant protection in the hepatic enzymatic activity but resveratrol was not found to be significantly effective (P ≤ 0.05). PB, TA and silymarin showed significant variation in the hepatic acid phosphatase activity when compared with resveratrol at 5% level. |
| |
| |
| Graph 2 (c-d) shows significant fall in the activity of alkaline phosphatase in liver and kidney after CCl4 administration (P ≤ 0.05). Therapy with PB, TA and resveratrol showed significant increase in the alkaline phophatase activity of liver (P ≤ 0.05). PB and TA treatment was equally effective in alkaline phosphatase activity, however resveratrol was found less effective. |
| |
| Adenosine triphosphatase |
| |
| Significant decline was observed in the activity of adenosine triphosphatase in liver and kidney after CCl4 administration as shown in (Graph 2 e-f). Post treatment with crude extract and tannic acid and resveratrol restored the depleted values and prevented the release of enzyme activity in both liver and kidney significantly (P ≤ 0.05). PB and TA showed better protective effect when compared with resveratrol by Tukey’s HSD post hoc test (P ≤ 0.05). |
| |
| Succinic dehydrogenase and catalase |
| |
| Administration of CCl4 caused significant decrease in the enzymatic activity of hepatic succinic dehydrogenase and catalase after 24 h of intoxication (Graph 3 a-b). Treatment with PB and TA showed significant improvement in the SDH activity. Therapy with resveratrol showed no significant improvement in the enzymatic activity. Tukey’s HSD post hoc test (P ≤ 0.05) clearly showed that resveratrol treatment was less effective when compared with TA and silymarin. Treatment with TA caused significant improvement in the enzymatic activity of hepatic catalase (P ≤ 0.05), while resveratrol and PB did not showed significant recovery [10]. |
| |
| Glycogen and glucose-6-phosphatase |
| |
| There was significant loss of the glycogen content and glucose-6- phosphatase activity in liver caused by CCl4 exposure (Graph 3 c-d). Parameters were recouped significantly with the PB extract, TA and resveratrol therapy (P ≤ 0.05) however the effect was more pronounced with tannic acid in glycogen (85.3%) and G-6-Pase (87.6%) after the CCl4 exposure. Therapy of TA showed significant improvement in the G-6-Pase activity when compared with resveratrol as analyzed by Tukey’s HSD post hoc test (P ≤ 0.05). |
| |
| Serum transaminases and alkaline phosphatase |
| |
| Table 1 summarizes the alterations in the enzymatic activities i.e. AST, ALT and SALP activities after acute acetaminophen induced intoxication. Treatments with PB extract and TA respectively showed protective effect by preventing the enhanced release of above parameters in serum towards control (P ≤ 0.05). Tannic acid (25 mg/kg, p.o.) showed its better therapeutic efficacy by recouping liver markers enzymes (AST, 83.7%; ALT, 80.5%; ALP, 82.1%) when compared with polygonum bistorta plant extract (AST, 61.4%; ALT, 69.9%; ALP, 66.2%). |
| |
|
|
Table 1: Data are mean ± S.E., N=6; @=Significant at P ≤ 0.05 for ANOVA; #APAP vs Control at P ≤ 0.05; *APAP+ Therapy vs APAP at P ≤ 0.05. Abbreviations: APAP=Acetaminophen; PB=Polygonum bistorta; TA=Tannic acid; S=Silymarin; % =Percent protection. |
|
| |
| Blood sugar, serum protein, albumin and bilirubin |
| |
| Table 2 demonstrated that the administration of APAP at a dose of 2 g/kg, once only exhibited significant (P ≤ 0.05) reduction in the blood sugar level whereas significant (P ≤ 0.05) elevation was observed in protein, albumin and bilirubin concentration in serum. Significant recoupment was seen with both therapeutic agents (P ≤ 0.05). However maximum improvement was seen with the therapy of tannic acid which is substantiated by the percent protection. |
| |
|
|
Table 2: Data are mean ± S.E., N = 6; @ =Significant at P ≤ 0.05 for ANOVA; #APAP vs Control at P ≤ 0.05; *APAP + Therapy vs APAP at P ≤ 0.05; Abbreviations: APAP = Acetaminophen; PB = Polygonum bistorta; TA = Tannic acid; S = Silymarin; %= Percent protection. |
|
| |
| Urea, creatinine, triglyceride and cholesterol |
| |
| Table 3 reveals a tremendous rise in the urea, creatinine, triglycerides and cholesterol contents after acetaminophen induced toxicity (P ≤ 0.05). Mean values of both therapies treated groups showed recoupment in all the parameters. However, tannic acid showed maximum protection and the values almost near to silymarin treated positive control group. Analysis of variance showed significant recoupment at 5% level in all the blood biochemical indices. |
| |
|
|
Table 3: Data are mean ± S.E., N = 6; @ = 76ySignificant at P ≤ 0.05 for ANOVA; #APAP vs Control at P ≤ 0.05; *APAP + Therapy vs APAP at P ≤ 0.05; Abbreviations: APAP = Acetaminophen; PB = Polygonum bistorta; TA = Tannic acid; S = Silymarin; % = Percent protection. |
|
| |
| Lipid peroxidation and reduced glutathione |
| |
| The lipid peroxide level in the tissues (liver and kidney) from APAP exposed group was significantly (P ≤ 0.05) higher than the respective control values. Therapeutic agents (PB extract and TA) showed marked reversal in the lipid peroxidation level. Curative treatment with plant extract prevents the significant alterations by recouping the values up to 50% in kidney but less effective in reducing the LPO level in liver (27%). However TA afforded better percent protection level between 50-65% in both the vital organs. Reduced glutathione is presumed to be an important endogenous defense against per oxidative destruction of cellular membranes. Thus, in the present study there was significant decline in the reduced glutathione level (P ≤ 0.05). Treatments with PB and TA recouped the glutathione concentration (P ≤ 0.05), which had been substantially decreased by APAP intoxication. Analysis of variance showed significant recoupment at 5% level in both the parameters. |
| |
| Histopathological Observations |
| |
| Light microscopic studies |
| |
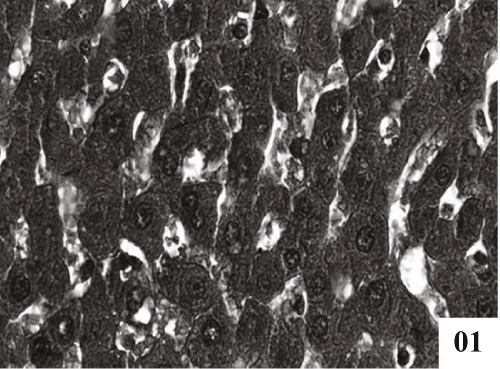
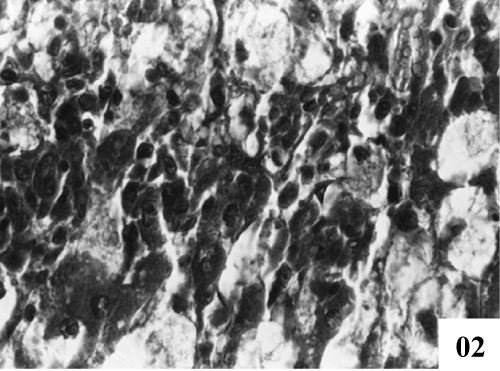
| Liver: Liver sections of normal control animals showed normal features (Figure 1). Histopathological studies after CCl4 administration demonstrated hydropic degeneration so liver cells showed swelling with optically empty cytoplasmic areas (Figure 2). Histopathological changes also included congestion in central veins, portal vessels, and sinusoids. |
| |
|
|
Figure 1: Photomicrographs of control rat liver showing maintained chord arrangement with well formed hepatocytes (X 400). |
|
| |
|
|
Figure 2: Administration of carbon tetrachloride: Photomicrographs showing centrilobular necrosis accompanied with fatty changes and ballooning hepatocyte were apparent (X 400). |
|
| |
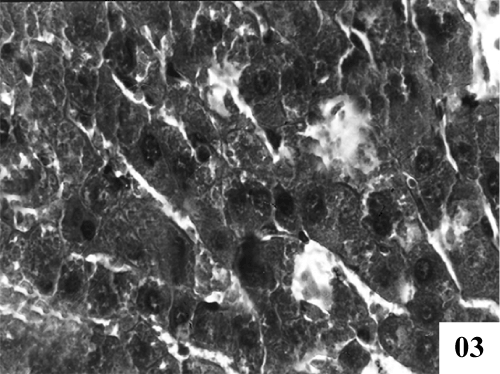
| Treatment with extract (100 mg/kg, p.o.) showed recoupment. Hepatocytes had angular appearance. Nuclei showed mild degeneration. The overall structure of hepatic lobules appeared normal except for few scattered degenerated cells (Figure 3). Post treatment with tannic acid showed significant hepatoprotection, exhibiting reduction in centrilobular necrosis, maintained chord arrangement (Figure 4). |
| |
|
|
Figure 3: Treatment of PB100 extract exhibiting reduction of centrilobular necrosis, however degeneration in some hepatocytes at some places were clearly visible mild improvement in sinusoidal dilatation was observed. (X 400). |
|
| |
|
|
Figure 4: Treatment with TA25 showing well formed trabecular structure of hepatocytes, clear sinusoidal spaces and nuclei (X 400). |
|
| |
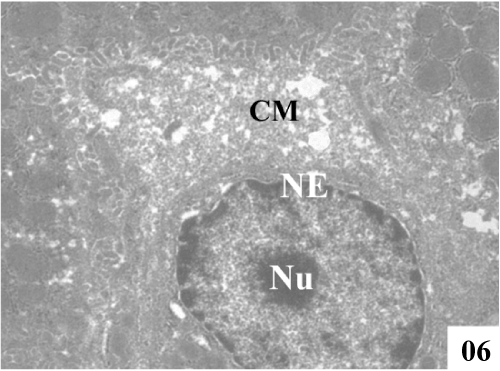
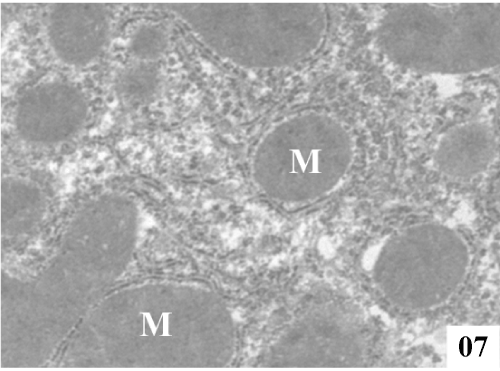
| Ultra structural changes: Electron micrographs demonstrated the main ultra structural features of the liver. The ultra structure of control rat liver showed hepatocytes separated by canaliculi and sinusoids (Figure 5). Hepatocytes were irregular in size and shape and had obscure boundaries separating them from the surrounding area, by which they can be distinguished from other cellular components. Pale foam like cytoplasm was seen. There was swelling of the mitochondria and disorganization of matrix in which the matrix density was reduced and the crystal tends to be sparse (Figure 6). |
| |
|
|
Figure 5: Electron micrographs of control rat liver showing prominent nucleus (N), conspicuous nucleolus (Nu), along with intact nuclear membrane (NM), with well formed extensive endoplasmic reticulum (ER) and plenty of mitochondria (M) with well formed cristae (X 4000). |
|
| |
|
|
Figure 6: Administration of CCl4 showed severe vacuolization, condensed cytoplasmic matrix (CM), damage in endoplasmic reticulum and swollen mitochondria with fused cristae (X 2800). |
|
| |
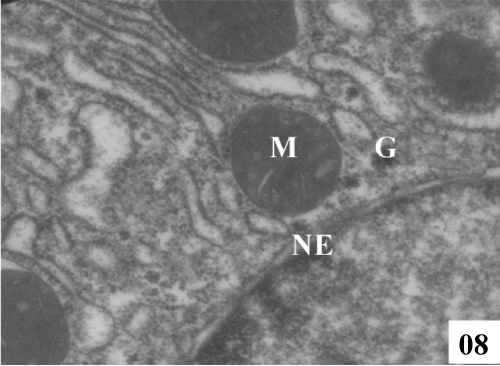
| Administration of the both therapeutic agents enhanced the process of regeneration. Liver sections treated with extract (100 mg/ kg, p.o.) showed nucleus was normal in appearance, nuclei envelopes were intact, majority of the mitochondria were normal. On the other hand, rats treated with tannic acid (25 mg/kg, p.o.) restored the ultra structure of hepatocytes which correlated with the improvement of liver function panel (Figures 7 and 8). |
| |
|
|
Figure 7: Treatment with PB100 showed well formed hepatocytes, along with rounded mitochondria (M) (8900). |
|
| |
|
|
Figure 8: Treatment with TA25 showed formed nucleus (N) with intact nuclear envelop (NE) along with rounded mitochondria (M), and glycogen rossets (G) (X 8900). |
|
| |
| Light microscopic studies |
| |
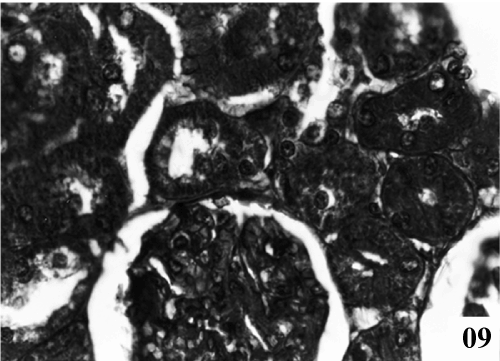
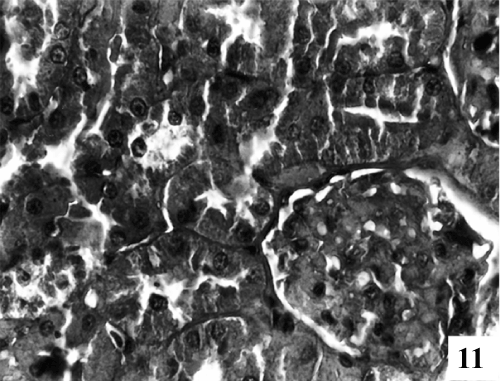
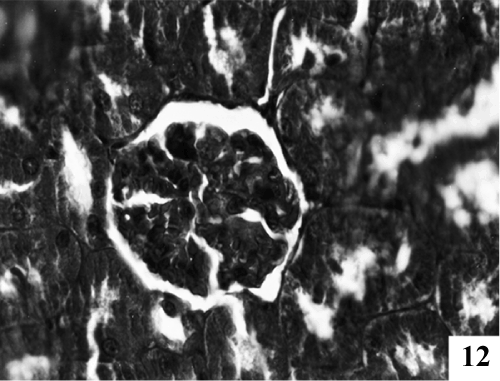
| Kidney: Kidney sections of control animals showed normal features (Figure 9). The structure of the kidney exposed to carbon tetra chloride exposure showed various degree of degeneration. Most of the epithelium lining of the collecting tubules had been sloughed into the lumen (Figure 10). Thus showing a toxic response on the structure of the kidney. According to microscopic examinations, severe lesions induced by CCl4 were remarkably reduced by the 5 days administration of the PB (100 mg/kg, p.o.) group, which were in good agreement with the results of the biochemical tests (Figure 11). Renal sections of animals in the TA 25 mg/kg showed the conspicuous recovery phenomenon. Therapy showed almost normal distal and proximal tubules, lumen was clear. The renal corpuscles appeared fairly normal. Glomeruli were compact with wide spacing in the Bowman’s capsule (Figure 12). |
| |
|
|
Figure 9: Photomicrographs of control rat kidney showing well formed renal corpuscles and Bowman’s capsule with compact glomeruli (X 400). |
|
| |
|
|
Figure 10: Photomicrographs of kidney exposed with CCl4 for to 21 days caused hypercellularity in glomeruli, severe degree of degeneration in renal tubules with eiosinophilic fluid (X 400). |
|
| |
|
|
Figure 11: Treatment with PB100 showing normal distal and proximal tubules with lumen was clear (X 400). |
|
| |
|
|
Figure 12: Treatment with TA25 showed well maintained Bowman’s capsule with well formed endothelial lining and well formed renal tubules. |
|
| |
| Comet assay |
| |
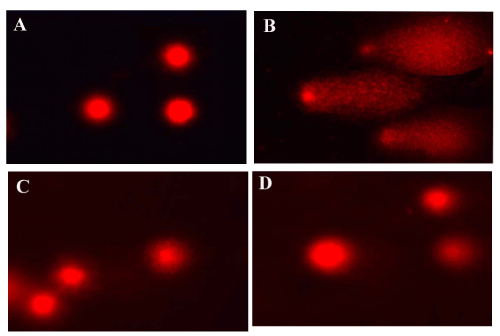
| The single-cell gel electrophoresis (comet assay) allows detection of DNA fragmentation in single cells, and was initially used for DNA damage estimation [11]. Figures 13 and 14 showed the effect of carbon tetrachloride on mean DNA damage, comet length, tail length and mean comet tail length. The damage was expressed as percent DNA migration in the tail and 20-25 nuclei were counted in each slide. In all CCl4 treated groups (1.5 ml/kg), the tail lengths were significantly increased in liver (42.6 μm) and kidney (74.6 μm). DNA damage was elevated the tail length and movement was significant high. Therapy with the active principle as tannic acid controlled DNA damage significantly as evident from the decrement of mean tail length when compared to Polygonum bistorta treated group. |
| |
|
|
Figure 13: Evaluation of DNA damage by comet assay in liver. |
|
| |
|
|
Figure 14: Evaluation of DNA damage by comet assay in kidney. |
|
| |
| MTT assay |
| |
| The cytotoxicity of P. bistorta and tannic acid toward in Hep2 cell lines was tested. The result showed that PB and TA concentrations of 10, 30 and 100 μg/ml were not toxic to the cell. Cytotoxicity was induced in Hep2 cell lines by exposure to 10 mM CCl4, and the cells were subsequently treated with PB and TA. As shown in table 4 tannic acid at concentrations of 10, 30 and 100 μg/ml significantly reduced cellular leakage of improved cell viability [12]. |
| |
|
|
Table 4: Data are mean ± S.E., N = 6; @ = Significant at P ≤ 0.05 for ANOVA #CCl4 vs C, *CCl4 + Therapy vs CCl4 at P ≤ 0.05; Abbreviations: C = Control; PB = Polygonum bistorta; TA = Tannic acid. |
|
| |
| Discussion |
| |
| Several animal experimental models have been in use to evaluate anti hepatotoxicants against classical hepatotoxicants viz. carbon tetrachloride and acetaminophen. The liver plays a major role in metabolism and has a number of functions in the body, including glycogen storage, decomposition of red blood cells, plasma protein synthesis, and detoxification. Many compounds, including clinically useful drugs, can cause cellular damage through metabolic activation of the chemicals to highly reactive compounds such as free radicals, carbenes and nitrenes [13]. Thus the purpose of this detailed investigation was to explore whether or not Polygonum bistorta and tannic acid could prevent the hepatic damage caused by CCl4 and acetaminophen, a model hepatotoxicants. |
| |
| Hepatic cells participate in a variety of metabolic activities and contain a lot of enzymes. The major intracellular enzymes are AST, ALT and ALP [14]. AST and ALT both are found in cytoplasm. AST particularly also exist in mitochondria whereas ALT is more abundant in liver cells. ALP mainly arises from the lining of the canaliculi and also from the sinusoidal surface of hepatocytes [15,16]. |
| |
| Therapy with Polygonum bistorta and tannic acid reversed the activity of transaminases and alkaline phosphatase activities thus restored them towards normal value depicting clearly improvement of the functional status of the liver cells thus showing marked hepatoprotective effect. In animals treated with TA there was no noticeable hepatocellular necrosis. Only mild ballooning & binucleate cells were observed in animals treated with PB (100 mg/kg) [3]. While binuclear cells were seen spread Binucleate cells in liver are indication of hepatic cell regeneration. In the present investigation there was a significant rise in urea and creatinine concentration after toxicant administration. It may be due to dysfunctional and dystrophic changes in the liver and kidney. Due to severe renal impairment, urea excretion falls and the serum concentration rise rapidly. |
| |
| Lipid peroxidation (LPO) is an autocatalytic process, which is a common consequence of cell death. It involves a broad spectrum of alterations, and the consequent degeneration of cell membranes may contribute towards the development of other disorders of lipoprotein metabolism, both in the liver and in peripheral tissues [17]. Glutathione-SH (GSH) is one of the most abundant tripeptide (y-glutamyl cysteinyl glycine) widely distributed in cells. A large reserve of reduced glutathione is present in hepatocytes and red blood cells. Liver is the major site for synthesis of GSH. The detoxification of different drugs and xenobiotics in the liver involves GSH. It is an intra-cellular reductant and plays major role in catalysis, metabolism and transport. Treatment with PB and TA shows recovery which is also supported by Phyllanthus niruri [18]. Proteins are synthesized in liver and inhibition indicates liver damage. A high bolus dose of acetaminophen results in significant loss of protein contents in liver. |
| |
| Treatment with Polygonum bistorta and tannic acid significantly ameliorates CCl4 induced changes in SDH and ATPase activities in liver and kidney of rats. The ameliorative effect of therapy might be due to its polyphenolic nature having antioxidative property. Antioxidants have the property to protect all membrane lipids and unsaturated fatty acids against oxidative degeneration. Similar investigative findings were also reported with the plant extracts of Phyllanthus fraternus [19] and Terminalia belerica Roxb [16]. |
| |
| Glycogen is the body's auxiliary energy source, tapped and converted back into glucose when there is need for energy. The maintenance of glycogen reserve is a specific function of the liver cell. Several enzymes are involved in the synthesis and degradation of glycogen. Disturbance of carbohydrate metabolism is one of the most significant biochemical lesions arising during CCl4 toxicity. G-6-Pase is a crucial enzyme of glucose homeostasis and plays an important role in the regulation of the blood glucose level [20]. Liver glycogen phosphorylase acts as the glucose sensor of liver, showing the breakdown of glycogen whenever the level of blood glucose is low. Subramanian and Selvam (1999) [21] reported that CCl4 caused significant decline in the G6Pase activity. |
| |
| This procedure has been widely used for genotoxicity studies [22] and for monitoring the exposure to DNA damaging agents in human populations. We assessed the extent of damage, which entailed measurement of comet length, tail movement, tail length and tail DNA (%) damage. Our data suggests that toxicant treatment was associated with oxidative stress induced DNA damage in liver and kidney. Therapy with PB extracts and tannic acid clearly prevented the densitometric and geometric parameters of the comets as determined using image analysis software. |
| |
| Protection of DNA damage by PB extract and TA clearly indicated its therapeutic potential at molecular level. HepG2 cell lines depicted favorable response in MTT assay. |
| |
| Acetaminophen is a widely used antipyretic and analgesic, produces acute liver damage if overdoses are consumed. Paracetamol is mainly metabolized in liver to excretable glucuronide and sulphate conjugates. However, the hepatotoxicity of paracetamol has been attributed to the formation of toxic metabolites when a part of paracetamol is activated by hepatic cytochrome P-450, to a highly reactive metabolite N-acetyl- P-benzoquinoneimine (NAPQI). NAPQI is initially detoxified by conjugation with reduced glutathione (GSH) to form mercapturic acid. However, when the rate of NAPQI formation exceeds the rate of detoxification by GSH, it oxidizes tissue macromolecules such as lipid or –SH group of protein and alters the homeostasis of calcium after depleting GSH. |
| |
| The experimental intoxication induced by carbon tetrachloride (CCl4) is widely used for modeling liver injury in rats [23]. Hepatotoxicity is connected with severe impairment of cell protection mechanisms. The location of liver injury is defined mainly by the biotransformation of CCl4, which is cytochrome P-450 dependant. Free radicals initiate the process of lipid peroxidation, which is generally caused of inhibition of enzyme activity. It is now generally accepted that the hepatotoxicity of CCl4 is the result of reductive dehalogenation, which is catalyzed by P-450, and which forms highly reactive trichloromethyl free radical. This readily interacts with molecular oxygen to form the trichloromethyl peroxy radical. Both trichloromethyl and its peroxy radical are capable of binding to proteins or lipids, or of abstracting a hydrogen atom from an unsaturated lipid, initiating lipid peroxidation and liver damage and by doing so playing a significant role in pathogenesis of diseases. These data provide a scientific explanation for the folkloric uses of Polygonum bistorta and tannic acid in the treatments of hepatic disorders. The findings provide a rationale for further studies on pharmacological evaluation. |
| |
| Acknowledgements |
| |
| The authors feel indebted to Prof. Sangeeta. Shukla and Prof. R. Mathur, School of Studies in Zoology, Jiwaji University, Gwalior for their invaluable suggestions. The financial assistance from Jiwaji University and Central Council of Research in Unani Medicine, New Delhi-India, is acknowledged. |
| |
| |
| References |
| |
- Suja V, Sharmila SL, Shyamala Devi C (1997) Protective effect of Liv.52 and Liv.100, ayurvedic formulation on lipid peroxidation in rat liver homogenate--an in vitro study. Indian J Exp Biol 35: 50-52.
- National Medicinal Plants Board (2008) Centrally Sponsored Scheme of National Mission on Medicinal Plants Operational Guidelines, Ayush, India.
- Duwiejua M, Zeitlin IJ, Gray AI, Waterman PG (1999) The anti-inflammatory compounds of Polygonum bistorta: Isolation and characterisation. Planta Med 65: 371-374.
- Harold Mc Gee (2004) On Food and Cooking: The Science and lore of the Kitchen. Simon & Schuster, New York 714.
- Rashid KA, Baldwin IT, Babish JG, Schultz JC, Mumma RO (1985) Mutagenicity tests with gallic and tannic acid in the Salmonella/mammalian microsome assay. J Environ Sci Health20: 153-165.
- Hain R, Bieseler B, Kindl H, Schröder G, Stöcker R (1990) Expression of a stilbene synthase gene in Nicotiana tabacum results in synthesis of the phytolaxin resveratrol. Plant Mol Biol 15: 325-335.
- Riley V (1960) Adaptation of Orbital Bleeding Technic to Rapid Serial Blood Studies. Proc Soc Exp Biol 104: 751-754.
- Snedecor GW, Cochran WG (1994) Statistical analysis (8th edn). Lowa State University Press: Ames, IA. 217-236.
- Yugarani T, Tan BK, Das NP (1993) The effects of tannic acid on serum and liver lipids of RAIF and RICO rats fed on high fat diet. Comp Biochem Physiol Comp Physiol 104: 339-343.
- Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47: 389-394.
- Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175: 184-191.
- Mossman TJ (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55-63.
- Gupta M, Mazumder UP, Kumar TS, Gomathi P, Kumar RS (2004) Antioxidant and hepatoprotective effects of Bauhinia racemosa against paracetamol and carbon tetrachloride induced liver damage in rats.Iranian J Pharmacol Therapeu 3: 12-20.
- Bhadauria M, Nirala SK, Shukla S (2008) Multiple treatment of propolis extract ameliorates carbon tetrachloride induced liver injury in rats. Food Chem Toxicol 46: 2703-2712.
- Chandan BK, Saxena AK, Shukla S, Sharma N, Gupta DK, et al. (2008) Hepatoprotective activity of Woodfordia fruticosa Kurz flowers against carbon tetrachloride induced hepatotoxicity. J Ethnopharmacol 119: 218-224.
- Jadon A, Bhadauria M, Shukla S (2007) Protective effect of Terminalia belerica Roxb. and gallic acid against carbon tetrachloride induced damage in albino rats. J Ethnopharmacol 109: 214-218.
- Tirkey N, Pilkhwal S, Kuhad A, Chopra K (2005) Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol 5: 1471-2210.
- Jalaluddin MI, Dewan FZ, Chowdhury SAR, Mamun MIR, Moshiuzzaman M, et al. (2007) Pre-treatment by n-hexane extract of Phyllanthus niruri can alleviate paracetamol-induced damage of the rat liver. Bangladesh J Pharmacol 2: 43-48.
- Padma P, Setty OH (1999) Protective effect of Phyllanthus fraternus against carbon tetrachloride-induced mitochondrial dysfunction. Life Sci 64: 2411-2417.
- Baginski ES, Foa PP, Zak B (1965) Glucose-6-phosphate. Hans Ulrich Bergmeyer, Jürgen Bergmeyer, Marianne Grassl, Methods of Enzymatic Analysis, 2nd Edition, Academy Press IncNew York 2: 876-880.
- Subramanian L, Selvam R (1999) Prevention of CCl4 - Induced hepatotoxicity by aqueous extract of turmeric. Nutr Res 19: 429-441.
- McKelvey-Martin VJ, Ho ET, Mckeown SR, Johnston SR, McCarthy PJ, et al. (1998) Emerging applications of the single cell gel electrophoresis (Comet) assay. I. Management of invasive transitional cell human bladder carcinoma. II Fluorescent in situ hybridization Comets for the identification of damaged and repaired DNA sequences in individual cells. Mutagenesis 13: 1-8.
- Gupta M, Mazumder UK, Thamilselvan V, Manikandan L, Senthilkumar GP, et al. (2007) Potential Hepatoprotective Effect and Antioxidant Role of Methanol Extract of Oldenlandia umbellatain Carbon Tetrachloride Induced Hepatotoxicity in Wistar Rats. IJPT 6: 5-9.
|
| |
| |