| Research Article |
Open Access |
|
| Prathiba Chichurakanahalli Srinivasan* |
| DNSVK, Sri Venkateshwara Dental College, Bangalore, India |
| *Corresponding authors: |
Prathiba CS
NSVK, Sri Venkateshwara Dental College
#25, 6th main, 13th ‘A’ Cross, Vyalikaval
Bangalore-560003, Karnataka, India
Tel: (+91) 9845485963
E-mail: csprati@yahoo.com |
|
| |
| Received July 23, 2012; Published August 31, 2012 |
| |
| Citation: Srinivasan PC (2012) Immunoglobulin Levels and Periodontal Diseases- A Clinical Immunological Study. 1: 254. doi:10.4172/scientificreports.254 |
| |
| Copyright: © 2012 Srinivasan PC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| There is little doubt that immunological mechanisms play an important role in the pathogenesis of periodontal diseases. Studies conducted so far have yielded contradicting results with regard to the immunoglobulin levels and also varying results after therapy. Hence, the present study was undertaken to study the levels of immunoglobulins-G, A and M in the serum and saliva of patients with periodontal disease. 40 systemically healthy subjects-10 cases of chronic periodontitis with 10 age and sex-matched controls and 10 cases of aggressive periodontitis with 10 age and sex-matched controls were included in the study. The serum and salivary Ig-G, A, and M levels were analyzed by immunoturbidimetry before and 6-8 weeks after Phase I therapy. In both the groups, there was increase in the immunoglobulin levels in cases compared to the controls, but individual variations were observed. There was a modest decline in the immunoglobulin levels after phase I therapy, but in some cases the levels increased after therapy. Therefore, further long-term studies with a larger sample population and with more definitive treatment procedures like periodontal surgery should be undertaken. |
| |
| Keywords |
| |
| Immunoglobulins; Periodontal diseases; Serum; Saliva |
| |
| Introduction |
| |
| Periodontal disease is considered to be a mixed infection wherein the pathogens act directly or indirectly in the destruction of the tooth-supporting tissues. The host reacts to this bacterial challenge by activating its defense mechanisms in an attempt to localize and eventually eliminate the pathogens [1]. The immune responses can be mediated either by antibodies (humoral) or by sensitized lymphocytes (cellular). |
| |
| Antibodies belong to the third fastest migrating group of serum globulins, the gamma globulins. The term Immunoglobulin (Ig) refers to the immunity-conferring portion of the gamma globulin fraction [2]. Based on physicochemical and antigenic differences, five classes of immunoglobulins have been recognized—IgG, IgA, IgM, IgD and IgE [3]. These immunoglobulins contribute to the inhibition of bacterial adherence and colonization, enhance bacterial phagocytosis, and help detoxify bacterial toxins and thus play a major role in the defense against bacterial infections [4]. The inflammatory and immune responses clearly contribute to the maintenance of homeostasis between the host and the microbial biofilm of the periodontium [5]. For the host to maintain homeostasis within the oral cavity, three distinct but interrelated immune responses contribute to controlling the microbial challenge. These are the salivary and gingival tissue (local) and the serum (systemic) immune systems [6]. |
| |
| According to Lehner, immunological responses (through local secretory and systemic serum antibodies) can be mediated by three related fluid compartments: Saliva, crevicular fluid and blood. Hence, immunoglobulins if present, should be detected in these fluid compartments [7]. |
| |
| Studies with evaluation of either serum or salivary quantitation of immunoglobulins have provided varying results. Some studies revealed increased serum IgG, IgA and IgM in patients with periodontitis [8- 14], while others showed no significant differences in serum Ig levels between periodontitis patients and healthy individuals [15-17]. A study conducted by Kaslick et al. [18] revealed increased levels of serum IgA, IgG and IgM in periodontosis patients, but paradoxically 41% of patients had no increase in IgG, IgA or IgM levels. |
| |
| Studies revealed increased salivary IgA in periodontitis patients [19-21], elevated salivary IgG and A levels in severe periodontitis patient [16], and another study showed salivary IgG and IgA to be elevated in juvenile periodontitis patients [22]. Contradicting these studies, study by Basu MK et al. [23] revealed decrease in salivary IgA in periodontitis patients compared to healthy individuals. |
| |
| Study by Bratthal GT and Ellen RP [24] revealed elevated salivary and crevicular antibodies to periodontal pathogens after conventional gingivitis treatment. Reiff RL [7] stated that levels of salivary and serum Ig G and A declined after Phase I therapy, but in the same study, some study subjects revealed elevations in the immunoglobulin levels after therapy. Basu MK et al. [23] observed higher salivary IgG and lower salivary IgA levels in periodontitis patients before oral hygiene therapy. The concentrations of these immunoglobulins after periodontal therapy was comparable with those found in clinically normal individuals. |
| |
| Since the above mentioned studies have yielded varying results, the present study is undertaken to estimate the total salivary and serum levels of Ig G, A and M in chronic and aggressive periodontitis cases followed by estimation of the same 6-8 weeks after phase I therapy and to compare the levels before and after phase I therapy. |
| |
| Materials and Methods |
| |
| Based on the criteria, 40 patients were recruited for the study. Since the study required collection of blood and saliva samples, written informed consent from patients and ethical clearance from the institution were obtained. |
| |
| Inclusion criteria |
| |
| 1. Male and female patients between 15 and 50 years of age. |
| |
| 2. Presence of loss of attachment and pocket probing depth greater than or equal to 4 mm prior to Phase I therapy. |
| |
| 3. Healthy individuals with no signs and/or symptoms of systemic disease. |
| |
| 4. Nonsmokers. |
| |
| 5. Individuals who have not undergone professional oral prophylaxis during the last one year. |
| |
| 6. Patients who have not received any antibiotic therapy 6 months prior to the commencement of the study. |
| |
| 7. Individuals included in the control group were systemically healthy individuals with good oral hygiene and no sign of periodontal disease, i.e., this group included subjects with a “healthy periodontium”—Gingival index (Loe and Silness 1963) of <0.5 and with no periodontal pockets exceeding 3 mm. |
| |
| 8. Patients who were compliant and willing to return after the Phase I therapy. |
| |
| Exclusion criteria |
| |
| 1. Patients who are suffering from any systemic diseases (e.g, diabetes mellitus, connective tissue disorders like rheumatoid arthritis). |
| |
| 2. Patients who are immunocompromised (e.g. HIV positive, primary and secondary immunodeficiency disorders, malnutrition etc). |
| |
| 3. Patients with a history of upper respiratory diseases of recent occurrence (within 4 weeks), allergic disorders or autoimmune disorders, and |
| |
| 4. Patients who are on corticosteriod medications or on cytotoxic drugs. |
| |
| A detailed medical and dental history was elicited from all the patients. The gingival index (Loe and Silness ) values were determined and recorded. The Shick and Ash Plaque index was used to assess the plaque. Probing pocket depth (gingival margin to the base of the gingival sulcus or pocket) was measured to the nearest mm on 6 sites per tooth using a William’s periodontal probe and recorded. Based on the clinical parameters assessed, the subjects were grouped as follows: |
| |
| Group A: Comprised of 20 systemically healthy subjects- 10 cases of chronic periodontitis and an equal number of age and sex-matched controls who had no evidence of periodontal destruction. |
| |
| Group B: Comprised of 20 systemically healthy subjects-10 cases of aggressive periodontitis and an equal number of age and sexmatched controls with no evidence of periodontal destruction. Before commencement of the treatment procedure, saliva and blood samples were collected from all the patients. |
| |
| Method of collection and storage of saliva samples |
| |
| The patients were informed not to eat or drink one hour before the collection of the saliva sample. Whole (mixed) resting/unstimulated saliva was collected in a sterile glass jar. About 3-4 ml of saliva was collected by the “draining or spitting method”. The subject was asked to accumulate saliva in the floor of the mouth and then expectorate saliva into a sterile glass jar. The saliva samples were centrifuged at 2500 g for 5 minutes to spin down the heavy mucous and other particles. The supernatant was pipetted into a sterile, dry screw-capped bottle and stored at –20 degree centigrade until it was analyzed. |
| |
| Method of collection of blood samples, preparation and storage of serum |
| |
| Blood was drawn by the Venipuncture technique from the antecubital fossa. The puncture site was cleaned with antiseptic and a tourniquet was placed around the upper arm 3-4 inches above the Venipuncture site to apply pressure and restrict the blood flow through the vein. A 5 ml syringe with a 21-gauge needle was used to draw about 2-3 ml of blood. Fasting blood samples were collected. The blood was transferred to a test tube and allowed to clot. The clotted blood samples were centrifuged at 3000 RPM for 10-15 minutes. The serum was pipetted into a sterile, dry screw-capped bottle and stored at -70 degree centigrade. |
| |
| Estimation of immunoglobulin concentrations |
| |
| Immunoturbidimetry, an automated procedure was employed to estimate the levels of immunoglobulins. The reagents provided in the kit that were specific for IgG, IgA and IgM were pipetted along with the serum and saliva samples separately for the estimation of IgG, IgA and IgM. The pippeting parameters specified by the manufacturer for each immunoglobulin was followed. The cassettes were then introduced into the THE HITACHI 704 ANALYSER. The results were obtained as a digital output on the computer monitor. |
| |
| Phase I therapy comprising of scaling and root planning was performed for the patients. Oral hygiene instructions were given. The patients were then dismissed and recalled after 6-8 weeks for reevaluation. The oral hygiene maintenance was assessed by the Shick and Ash Plaque index (score less than 1.0). If the patients had achieved a good level of plaque control then the samples of saliva and blood were collected again and analyzed for the levels of immunoglobulins. |
| |
| Results |
| |
| In the chronic periodontitis group, the serum IgG level in 8 out of the 10 cases was higher than their controls, in 2 cases below the levels in the control, but still within the normal range. After therapy, the levels increased in 4 out of the 10 cases (Table1). |
| |
|
|
Table 1: Serum IgG levels (mg/dl) in Chronic Periodontitis patients before and after therapy and in their controls. |
|
| |
| In 8 out of the 10 cases, the serum IgA levels were higher than the controls and in 2 cases lower than the controls. After therapy, the levels increased in 3 out of the 10 cases (Table 2). In 8 out of the 10 cases, the serum IgM levels were higher than the controls and in 2 cases the IgM levels were lower than the controls-in one patient the level was below the normal range. After therapy, the levels increased in 3 cases (Table 3). |
| |
|
|
Table 2: Serum IgA levels (mg/dl) in Chronic Periodontitis patients before and after therapy and in their controls. |
|
| |
|
|
Table 3: Serum IgM levels (mg/dl) in Chronic Periodontitis patients before and after therapy and in their controls. |
|
| |
| In 9 cases, the salivary IgG levels were higher than the controls. In one case, the level was lower than the control. After therapy, the levels were increased in 5 cases (Table 4). In one case, the level of salivary IgA was lower than the control levels. After therapy, increased levels were observed in 3 cases (Table 5). In one case, the salivary lgM level was lower than the control. After therapy, the levels were increased in 3 cases (Table 6). |
| |
| |
|
|
Table 4: Salivary IgG levels (mg/dl) in Chronic Periodontitis patients before and after therapy and in their controls. |
|
| |
|
|
Table 5: Salivary IgA levels (mg/dl) in Chronic Periodontitis patients before and after therapy and in their controls. |
|
| |
|
|
Table 6: Salivary IgM levels (mg/dl) in Chronic Periodontitis patients before and after therapy and in their controls. |
|
| |
| In the aggressive periodontitis group, the serum IgG levels in one case was below the control, but still within the normal range. After therapy, the levels increased in 4 cases (Table 7). The IgA levels in one case, was below the levels in the control, but still within the normal range. After therapy, the levels increased in 3 cases (Table 8). In all the 10 cases, the IgM levels were higher than the controls. After therapy, the levels were raised in 5 cases (Table 9). The Salivary IgG in all the 10 cases was higher than in the controls. After therapy, the levels were increased in 3 cases (Table 10). Likewise the salivary IgA and IgM levels in all the cases were higher than the controls. After therapy, the levels were raised in 2 cases (Table 11 and Table 12 respectively). |
| |
|
|
Table 7: Serum IgG levels (mg/dl) in Aggressive Periodontitis patients before and after therapy and in their controls. |
|
| |
|
|
Table 8: Serum IgA levels (mg/dl) in Aggressive Periodontitis patients before and after therapy and in their controls. |
|
| |
|
|
Table 9: Serum IgM levels (mg/dl) in Aggressive Periodontitis patients before and after therapy and in their controls. |
|
| |
|
|
Table 10: Salivary IgG levels (mg/dl) in Aggressive Periodontitis patients before and after therapy and in their controls. |
|
| |
|
|
Table 11: Salivary IgA levels (mg/dl) in Aggressive Periodontitis patients before and after therapy and in their controls. |
|
| |
|
|
Table 12: Salivary IgM levels (mg/dl) in Aggressive Periodontitis patients before and after therapy and in their controls. |
|
| |
| Discussion |
| |
| It is pretty much agreed that the immune system is involved in the pathogenesis of periodontal disease. The literature is replete with studies involving the immunoglobulin levels in different forms of periodontal diseases and these studies have yielded varying results. |
| |
| In the present study, individual variations have been observed with regard to the immunoglobulin levels, i.e., although the serum and salivary levels are elevated in most of the cases, there are some exceptions where the levels were lesser than the controls, but still within the normal range. After therapy, in some cases there was a decline in the levels while in some others there was an increase. |
| |
| The data collected in the study was analyzed statistically, by computing the necessary statistics like mean, standard deviation, standard error of mean, and 95% confidence interval for mean. Unpaired student t-test (** in Tables 13-16) is employed to compare immunoglobulin levels between the cases and controls and paired t-test (* in Tables 13-16) is used to compare the immunoglobulin levels in the cases before and after therapy. The results are considered statistically significant whenever p ≤ 0.05. |
| |
|
|
Table 13: Statistical inference based on t-test of disease-Chronic Periodontitis (serum). |
|
| |
|
|
Table14: Statistical inference based on t-test of disease-Chronic Periodontitis (saliva). |
|
| |
|
|
Table15: Statistical inference based on t-test of disease-Aggressive Periodontitis (serum). |
|
| |
|
|
Table 16: Statistical inference based on t-test of disease-Aggressive Periodontitis (saliva). |
|
| |
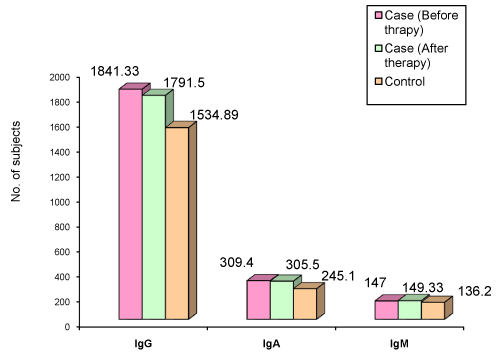
| Figure 1 and Table 13 summarizes the serum immunoglobulin levels of chronic periodontitis cases before and after therapy. The levels of IgG (p<0.033) and IgA (p<0.002) were significantly higher than in the controls. The IgM levels were not significantly raised in the cases. (p >0.482). This finding is in contrast to the results of the studies conducted by Tortelli A et al. [10], Seidlova et al. [12] and Anil S et al. [13] but is in agreement with the study conducted by Bokor-Bratic M [17]. The probable cause for increased IgG levels may be due to their increased production to neutralize bacterial toxins. |
| |
|
|
Figure 1: Mean distribution of IgG, IgA, and IgM in serum of Chronic Periodontitis Cases. |
|
| |
| After therapy, there was no significant decline in the serum levels of IgG (p>0.202) and IgA(p> 0.323), these results in agreement with the results of Reiff RL [7] and Papapanou et al. [25] for IgG, (the latter study concluded that despite successful periodontal therapy, titers remained elevated over a 30-month period) and in agreement with the study of Reiff RL [7] for IgA. There was no significant decline in IgM levels after therapy (p>0.665). The mean IgM value before therapy was 147.00 and the value after therapy was 149.80. In 3 of the 10 of the cases the levels increased after Phase I therapy. |
| |
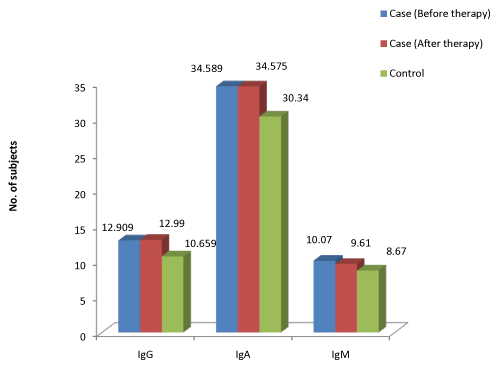
| Figure 2 and Table 14 summarizes the levels of salivary immunoglobulins in chronic periodontitis cases before and after therapy. The salivary IgG levels in the cases was not significantly higher than in the controls (p>0.510). This is in contrast to the results obtained in the study conducted by Basu MK et al. [23]. The salivary IgA levels is significantly higher than in the controls (p<0.059). This finding correlates with the results of the study conducted by Guven et al. [19], Lindstrom et al. [20] and Nagasawa T et al. [21]. IgM levels in saliva of patients is significantly higher than the controls (p<0.024). This result is in contrast to the study conducted by Yavuzyilmaz E et al. [26]. |
| |
|
|
Figure 2: Mean distribution of IgG, IgA, and IgM in saliva of chronic periodontitis cases. |
|
| |
| Enzymes that cleave the IgG-the proteases, hypothesized as important virulence factors of bacterial pathogens [27]. This may be the reason for the decline in the IgG levels. Prolonged antigenic stimulus in an infectious condition like periodontal disease may also stimulate the local IgA immune system [20]. Local immunoglobulin synthesis in the inflamed gingiva in periodontitis patients produces mainly IgG and IgA, not IgM. Thus the elevated IgM levels may be due to the raised level of glandular secretory output or from increased leakage from the blood via the gingival sulcus [20]. |
| |
| The mean level of salivary IgG before therapy is 12.91 and after therapy is 12.99 (p>0.853). In 3 out of the 10 of the cases the levels of salivary IgG and IgA (p>0.994) increased after Phase I therapy. This finding is in agreement with the results obtained in the study conducted by Reiff RL [7], where there was a reduction in levels in most of the cases, but there was increase in levels as well in few cases following Phase I therapy. The results are in contrast to the results of the study conducted by Basu MK et al. [23]. There was a statistically significant decline in the salivary IgM levels in patients following Phase I therapy (p<0.035). The most probable reason for this is the reduction in the acute phase of the infection. |
| |
| Increase in the salivary Ig G, A and M levels in some cases after therapy can be attributed to the the following causes: Scaling itself may cause a transient rise in the blastogenic response [7] or inoculation of the microorganism into the host tissues resulting from scaling can lead to elevated titers, or even elimination of the immunosuppressive microorganism can lead to elevated levels after therapy [28]. |
| |
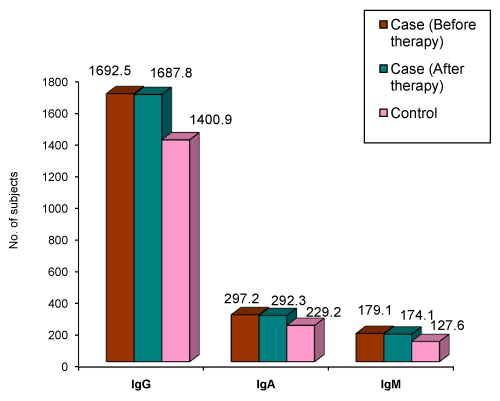
| Figure 3 and Table 15 summarizes the immunoglobulin levels in serum of aggressive periodontitis cases before and after therapy. Serum IgG levels is significantly higher than the levels in controls (p<0.007). This finding correlates with the results obtained in the studies of Lehner et al. [9], Kaslick RS et al. [18], Johnson RJ et al. [11], and Ranney RR et al. [16]. The serum IgA (p<0.020) and the IgM (p<0.0001) is significantly higher than the levels in controls. This finding correlates with the results obtained in the studies of Lehner et al. [9] and Kaslick RS et al. [18] for IgA, and the study by Lehner et al. [9] for IgM. |
| |
|
|
Figure 3: Mean distribution of IgG, IgA, and IgM in serum of Aggressive Periodontiits cases. |
|
| |
| The elevation in serum IgG levels may be due to the increased antibody production to neutralize bacterial toxins. Brandtzaeg and Kraus reported an increased IgA content of the inflamed gingival, which may be the reason for the increased levels of IgA [13]. The increased levels of serum IgM may reflect a response to the gramnegative bacteria, most commonly associated with periodontitis [9]. |
| |
| After therapy, the decline in the serum IgG (p>0.758) and IgA (p>0.137) levels in the cases was not statistically significant. This finding is in agreement with the results of the study obtained by Reiff RL [7]. Likewise, there was no significant decline in the serum IgM levels (p>0.123) after therapy. |
| |
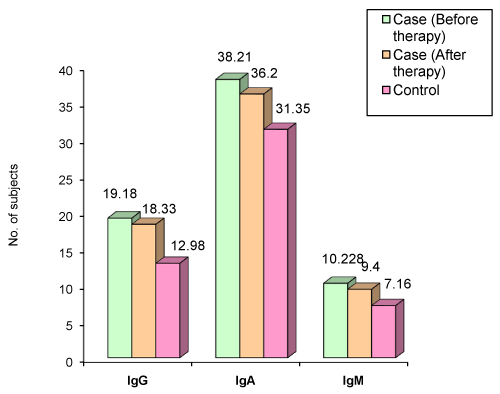
| Figure 4 and Table 16 summarizes the levels of salivary immunoglobulins in aggressive periodontitis patients before and after phase I therapy. The salivary IgG levels (p<0.012) in the cases is significantly higher than the controls and is in agreement with the studies conducted by Ranney RR et al. [16], Sandholm L et al. [22]. The above finding is in contrast to the results obtained in the study conducted by Saxen L et al. [29]. The salivary IgA levels in cases is significantly higher than the controls (p<0.04). This finding is in agreement with the results of the study conducted by Ranney RR et al. [16], but contradicts the results of study by Sandholm et al. [22] and Saxen L et al. [29]. The salivary IgM levels is significantly higher than the controls (p<0.001). This finding correlates with the results obtained in the study conducted by Sandholm et al. [22], but is in contrast to the results obtained by Saxen L et al. [29]. |
| |
|
|
Figure 4: Mean distribution of IgG, IgA, and IgM in saliva of aggressive periodontitis cases. |
|
| |
| The increased levels of IgG in saliva of patients with aggressive periodontitis may be due to the predominating synthesis of IgG and the transfer of this and serum-derived IgG from the gingival tissue to the oral cavity. The increased levels of IgA in whole saliva of patients with aggressive periodontitis may be due to the increased leakage of the serum via the inflamed pocket epithelium. Prolonged antigenic stimulus in an infectious condition like periodontal disease may also stimulate the local IgA immune system [7]. Elevated IgM levels may be due to the raised level of glandular secretory output or from increased leakage from the blood via the gingival sulcus [22]. |
| |
| After therapy, although there was a decline in the salivary IgG levels, it was not statistically significant (p>0.679). This is in agreement with the study conducted by Reiff RL [7]. |
| |
| However, the decline in the salivary IgA (p<0.044) and IgM (p<0.036) was statistically significant. This finding is in contrast to the results of the study conducted by Reiff RL [7]. One of the possible causes for this may be the individual patient variation with respect to the oral microflora present at the time of sampling, the varying degrees of periodontal pathology and varying degrees of inflammation present at the site. |
| |
| The role of immunoglobulins in the pathogenesis of periodontitis is not clear. Several questions remain unanswered. At what stage in the infection is the antibody detected? i.e., it is not clear at what point in the infection and subsequent disease process the initial seroconversion occurs. Once detected should it be considered as a sign of improvement in the condition or decline in the condition? How is the antibody associated with active disease? and Can early immune responses be detected prior to gross infection to enable early institution of therapeutic modalities? Also, the effects of therapy on the levels of immunoglobulins are not clear. Whether the raise in the immunoglobulin levels after Phase I therapy induced by the scaling procedure is beneficial is unanswered. The long-term study of the disease process from its inception and in its various stages may provide answers to the questions raised. |
| |
| Hence, further long-term studies with a larger sample population and with advanced immunological techniques have to be undertaken to study the role played by the immunoglobulins in the pathogenesis of periodontitis, to define at-risk population, and to use immunological data for diagnosis, classification and monitoring of periodontal diseases. Long-term follow-up studies will also shed light on the changes in the immunoglobulin levels following various treatment modalities employed for treatment of periodontal diseases. |
| |
| |
| References |
| |
- Landi L, Amar S, Polins AS, Van Dyke TE (1997) Host mechanisms in the pathogenesis of periodontal disease. Curr Opin Periodontol 4: 3-10.
- Sahingur SE, Cohen RE (2004) Analysis of host responses and risk for disease progression. Periodontol 2000 34: 57-83.
- Ananthanarayan R, Paniker CKJ (1990) Antibodies-Immunoglobulins. In: Textbook of Microbiology. (4th edn), Orient Longman Ltd, Madras, PP: 84-91.
- Albandar JM, DeNardin AM, Adesanya MR, Winn DM, Diehl SR (2002) Associations of serum concentrations of IgG, IgA, IgM and interleukin-1beta with early-onset periodontitis classification and race. J Clin Periodontol 29: 421-426.
- Ebersole JL, Holt SC, Capelli D, Singer RE (1998) IgA antibody responses to periodontal pathogens in non-human primates. Symposium proceedings-IgA and Periodontal Diseases. Abstracts of IADR symposium Nice, France.
- Ebersole JL (1996) Immune responses in Periodontal Diseases. In: Wilson TG, Kornman KS (eds) Fundamentals of Periodontics. Quintessence Publishing Co Inc, Illinois PP: 109-158.
- Reiff RL (1984) Serum and salivary IgG and IgA response to initial preparation therapy. J Periodontol 55: 299-305.
- Carvel RI, Halperin V, Wallace JH (1973) Immunological studies in chronic severe alveolar resorptive disease: a report of two young female patients. J Periodontol 44: 25-34.
- Lehner T, Wilton JM, Ivanyi L, Manson JD (1974) Immunological aspects of juvenile periodontitis (periodontosis). J Periodontal Res 9: 261-272.
- Törteli A, Backhausz R, Bruder S (1975) [Electrophoretic study of serum proteins in patients with periodontal diseases]. Stomatol DDR 25: 312-316.
- Johnson RJ, Matthews JL, Stone MJ, Hurt WC, Newman JT (1980) Immunopathology of periodontal disease. I. Immunologic profiles in periodontitis and juvenile periodontitis. J Periodontol 51: 705-712.
- Olśanska-Seidlová A, Skarlandt P, Mikulecky M, Seymour G (1989) Some immunological findings in adult periodontitis. Aust Dent J 34: 417-420.
- Anil S, Hari S, Remani P, Vijaykumar T (1990) Immunology of chronic generalized periodontitis. 1. Estimation of cellular and humoral immune status. Indian J Dent Res 2: 127-132.
- Wilton JM, Hurst TJ, Sterne JA, Caves J, Tilley C, et al. (1992) Elevated levels of the IgG2 subclass in serum from patients with a history of destructive periodontal disease. A case-control study. J Clin Periodontol 19: 318-321.
- Schenkein HA, Genco RJ (1977) Gingival fluid and serum in periodontal diseases. I. Quantitative study of immunoglobulins, complement components, and other plasma proteins. J Periodontol 48: 772-777.
- Ranney RR, Ruddy S, Tew JG, Welshimer HJ, Palcanis KG, et al. (1981) Immunological studies of young adults with severe periodontitis. I. Medical evaluation and humoral factors. J Periodontal Res 16: 390-402.
- Bokor-Bratić M (1998) The concentration of immunoglobulins A, G, and M in serum of patients with periodontal disease. Med Pregl 51: 310-314.
- Kaslick RS, West TL, Singh SM, and Chasens AI (1980) Serum immunoglobulins in Periodontosis patients. J Periodontol 51: 343-344.
- Güven O, De Visscher JG (1982) Salivary IgA in periodontal disease. J Periodontol 53: 334-335.
- Lindstrom FD, Folke LEA (1973) Salivary IgA in Periodontal disease. Acta Odontol Scand 31: 31-34.
- Nagasawa T, Aramaki M, Ishikawa I (1999) The role of salivary IgA antibody against periodontopathic bacteria. Symposium proceedings-IgA and Periodontal Diseases. Abstracts of IADR symposium 26 June 1998, Nice, France.
- Sandholm L, Grönblad E (1984) Salivary immunoglobulins in patients with juvenile periodontitis and their healthy siblings. J Periodontol 55: 9-12.
- Basu MK, Fox EC, Becker JF (1976) Salivary IgG and IgA before and after periodontal therapy. A preliminary report. J Periodontal Res 11: 226-229.
- Tynelius-Bratthall G, Ellen RP (1985) Fluctuations in crevicular and salivary anti-A. viscosus antibody levels in response to treatment of gingivitis. J Clin Periodontol 12: 762-773.
- Papapanou PN, Neiderud AM, Disick E, Lalla E, Miller GC, et al. (2004) Longitudinal stability of serum immunoglobulin G responses to periodontal bacteria. J Clin Periodontol 31: 985-990.
- Yavuzyilmaz E, Yamalik N, Calguner M, Ersoy F, Baykara M, et al. (1992) Clinical and immunological characteristics of patients with rheumatoid arthritis and periodontal diseases. J Nihon Univ Sch Dent 34: 89-95.
- Gregory RL, Kim DE, Kindle JC, Hobbs LC, Lloyd DR (1992) Immunoglobulin-degrading enzymes in localized juvenile periodontitis. J Periodontal Res 27: 176-183.
- Miyasaki KT (1996) Altered leukocyte functions and periodontal disease. In: Haake SK (ed) Clinical Periodontology. (8th edn) W.B. Saunders company, Philadelphia PP: 132-150.
- Saxén L, Tenovuo J, Vilja P (1990) Salivary defense mechanisms in juvenile periodontitis. Acta Odontol Scand 48: 399-407.
|
| |
| |