| Research Article |
Open Access |
|
| Najmul Hasan1*, Farhan Ahmed Siddiqui2, Nawab Sher Afridi2, Mathurot Chaiharn3, Sauleha Khan2, and Mohammed Abrar2 |
| 1Department of Microbiology, Faculty of Science, University of Karachi, Pakistan |
| 2Department of Chemistry, Faculty of Science, University of Karachi, Pakistan |
| 3Department of Biology, Faculty of Science, Maejo University, Thailand |
| *Corresponding authors: |
Najmul Hasan
Department of Microbiology
Faculty of Science, University of Karachi
Karachi, Pakistan 75270
Tel: 0092-3333669331
E-mail: najam.edu.pak@gmail.com |
|
| |
| Received August 10, 2012; Published August 30, 2012 |
| |
| Citation: Hasan N, Siddiqui FA, Afridi NS, Chaiharn M, Khan S, et al. (2012) A New Acetonitrile-Free, Cost-Effective, Simple and Validated Rp-HPLC Method for Determination of Montelukast Sodium in Bulk, Tablets and Liquid Dosage Forms. 1: 261. doi:10.4172/scientificreports.261 |
| |
| Copyright: © 2012 Hasan N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| A new accurate, specific, precise, acetonitrile-free and cost-effective high performance liquid chromatographic method has been developed and validated for the determination of montelukast in bulk and in its pharmaceutical dosage forms of tablet and suspension. Methanol: D.I water adjusted to pH 2.8 ± 0.1 was used as the mobile phase at a flow rate of 1.0 ml/min using a Symmetry C18 column. The effluent was spectrophotometrically monitored at 220 nm. The intraday and inter-day precisions showed coefficients of variation ranging from 0.54% to 1.14% at three concentrations. The calibration curve was linear with a correlation coefficient of 0.999979. The averages of the absolute and relative recoveries were found to be 98.78 to 99.25%. The proposed HPLC method was successfully applied to quantify the amount of montelukast in bulk and two dosage forms in quality control. |
| |
| Keywords |
| |
| Montelukast; Acetonitrile free; Methanol; RP-HPLC |
| |
| Introduction |
| |
| Montelukast (MKT) ([R-(E)]-1[[[1-[3-[2-(7-chloro-2-quinolinyl) ethyl] phenyl]-3-[2-(1-hydroxy-1-methylethyl) phenyl] propyl]thio] methyl] cyclo propane acetic acid, (Figure 1) is a selective and orally active cysteinyl leukotriene (CysLT 1 ) receptor antagonist. It is used for prophylaxis and chronic treatment of asthma in children and adults [1- 6]. Montelukast, a leukotriene modifier, has clearly demonstrated the ability to ameliorate bronchoconstriction and indices of airway edema and abnormal mucus production as observed in clinical trials [7]. |
| |
|
|
Figure 1: It shows chemical structures of Montelukast (Left) and Montelukast Sodium (Right). |
|
| |
| Several analytical methods have been reported for the determination of Montelukast alone or in combination with other drugs and commercial pharmaceutical products, using different spectrophotometric methods including some HPLC methods using ultraviolet (UV) or mass spectrophotometric (MS) detection alone. Few liquid chromatographic methods with UV detection [8], fluorescence detection [9,10], stereo selective determination of S-enantiomer of montelukast on column switching technique with fluorescence detection [11], column switching HPLC [12] semi-automated 96-well protein precipitation [13,14] are reported for the assay of montelukast in human plasma. Other techniques include pressurized liquid extraction followed by HPLC [15] and LC-MS methods [16-18]. The reported methods are based on either protein precipitation techniques, which are preliminary in nature and nonspecific [19] or solid phase extraction technique. |
| |
| But these methods may not always be suitable due to several reasons. Montelukast is a very common drug available in many commercial pharmaceutical products either alone or in combination with other drugs. Therefore still there was a need for a simple and sensitive analytical method for Montelukast in bulk drug, commercial products and biological samples. Keeping this point into consideration, an attempt was made to develop a simple, sensitive and validated stability indicating RP-HPLC method using UV detection. The applicability of the method was confirmed for analysis of Montelukast in pharmaceutical products (tablets and suspension). The results of analysis were validated in accordance with ICH guidelines [20]. |
| |
| Experimental |
| |
| The present method was designed to be easy to use, sensitive, rapid and cost effective with simple sample preparation for Montelukast sodium. Separation and quantification of Montelukast sodium in pharmaceutical drug formulations are achieved with an isocratic elution. |
| |
| Material and reagents |
| |
| Montelukast sodium was kindly gifted by AGP (Private) Limited, Phosphoric acid Merck, (Germany), Methanol (HPLC grade) from Fisher Scientific (UK). The suspension and tablets containing Montelukast sodium was obtained from commercial source (Lucast®, Aerokast®, Beasy®, and Lucast suspension®) labeled to contain 10 mg/tab and 1 mg/ml respectively. Distilled and deionized water was obtained by passage through RO plant (Waterman, Pakistan) and was further filtered through a 0.45 μm membrane filter (Millipore, Bedford, MA, USA). |
| |
| Chromatographic conditions |
| |
| The HPLC analysis was carried out at ambient temperature. The compound was chromatogrphed isocratically with a mobile phase consisting of Methanol (HPLC grade): deionized water (90:10, % v/v) with the pH adjusted to 2.8 ± 0.1 using phosphoric acid. The mobile phase was filtered by passing through a 0.45 μm membrane filter (Millipore, Bedford, MA, USA).The flow rate was 1.0 ml/min, and the injected volume was 20 μL. The effluent was monitored spectrophotometrically at a wavelength of 220 nm. |
| |
| Apparatus |
| |
| For chromatography we used a SIL 10A auto injector HPLC system comprising of SCL 10A system controller, SPD 20A prominence UV/ VIS detector, with a Shimadzu LC 20 AT pump with LC Solutions software. Separation was performed on a Hyperpack ODS C18 HPLC column, (4.6×250 mm; 5 μm bead size) maintained at ambient temperature 25 °C, Ultrasonic cleaner (Elmasoni E 60 H), Jenway 3240 pH meter and Sartorious TE2145 analytical balance. Throughout the work only amber glass flasks were used to avoid light effect on the solution of montelukast standards and samples. |
| |
| Analytical Procedure |
| |
| Standard preparation |
| |
| In a 100 ml volumetric flask, weighed accurately about 20.8 mg of Montelukast sodium reference standard. Dissolve up to 50 ml in Methanol (HPLC Grade) sonicate for 10 minutes let it cool to room temperature and make up volume with the extraction solvent stir well for 20 minutes and diluted 2.5 ml in a 50 ml volumetric flask to get 10 μg/ml working standard solution of Montelukast base. Filter through 0.45 micron filter paper. This solution can be used for 3 days, if stored protected from light |
| |
| Sample preparation |
| |
| |
| Analysis of tablets: For making sample of 10 μg/ml Montelukast, 20 tablets were weighed and ground to get an evenly homogenized powder. The sample was weighed accurately equivalent to 10 mg of Montelukast and taken in 100 ml volumetric flask and 50 ml of extraction solvent was added. The sample was sonicated for 10 minutes and the placed for stirring for 10 minutes to cool down the temperature and then added extraction solvent up to the mark. The solution was diluted in a 50 ml volumetric flask to get 10 μg/ml working standard solution by adding 5.0 ml of stock solution. The sample was then filtered through 0.45 mm filter paper and injected into the HPLC system. |
| |
| Analysis of suspension: To prepare a sample of 10 μg/ml Montelukast from suspension, the suspension was shaken well before and was accurately weighed as 11.1gram (density, 1.1 g/ml) equivalent to 10 mg of Montelukast. The sample was taken in 100 ml volumetric flask and 50 ml of extraction solvent was added. The sample was sonicated for 20 minutes and then placed for stirring for 30 minutes to cool down the temperature and then added extraction solvent up to the mark. Then 5ml was diluted in 50 ml further. The sample was then filtered and injected into the HPLC system. |
| |
| Method Development |
| |
| For developing an efficient method for the analysis of MKT, parameters, such as detection wavelength, mobile phase proportions, optimum pH and concentration of the standard solutions were comprehensively studied. Mobile phase was selected in terms of its components and proportions. Then chromatographic parameters were evaluated using a Symmetry C18 column and a mobile phase composed of Methanol and water (80:20, v/v). In spite of achieving a good result, this condition exhibited a long run time, since Montelukast peak was eluted after 19 min. Hence, different ratios of methanol and water (82: 18, 84: 16, 86: 14, 88: 12, 90: 10, 92: 08 and 94: 06) were tried to obtain an adequate and representable result. The mobile phase composed of Methanol and water (90:10, v/v) promoted a short run time (7 min) without any interference, so this condition was adopted in sub-sequent analyses. |
| |
| Method Validation |
| |
| The method validation was performed in accordance with ICH guideline. For the assay of Montelukast sodium various procedures were performed including system suitability, specificity, linearity, range, accuracy, intra-day and inter-day precision, limit of detection, limit of quantification and robustness [21,22]. |
| |
| Linearity and Range |
| |
| To study the linearity of standard solutions of Montelukast at different concentration levels, 5 dilutions were prepared to give standards solution of 50, 100, 150 and 200 %. The standard calibration curve was generated using regression analysis with Microsoft excel (Table 1). |
| |
|
|
Table 1: Calibration curve data and validation parameters. |
|
| |
| Specificity |
| |
| Specificity is a procedure to detect quantitatively the analyte in the presence of component that may be expected to be present in the sample matrix. Commonly used excipients in tablet and suspension preparation were spiked in a pre-weighed quantity of drugs and then absorbance was measured and calculations done to determine the quantity of the drugs. |
| |
| Precision |
| |
| Precision was studied to find out intra and inter day variations in the test methods of Montelukast in the concentration range of 8-12 μg/ ml for three times on the same day and three different days. Precision was determined by analyzing corresponding standard daily for a period of three days. Analysis was performed at three different days with three separate samples from same homogeneous bulk. |
| |
| Accuracy |
| |
| The montelukast reference standard was accurately weighed and added to a mixture of the tablet and suspension excipients, at three different concentration levels (8, 10 and 12 μg/ml of Montelukast). At each level, samples were prepared in triplicate and the recovery percentage was determined. |
| |
| LOQ and LOD |
| |
| Standard solutions were prepared by sequential dilutions at decreasing concentrations, in the range of 10-1.25 ng /ml of Montelukast and injected onto the chromatograph. |
| |
| Robustness |
| |
| The robustness was studied by analyzing the same samples of MKT by deliberate variation in the method parameters. Doing small changes in the chromatographic conditions like mobile phase, flow rate etc. and change in the responses of MKT was noted. For this purpose changing in the extraction time of MKT from dosage forms by ± 2 min, composition of mobile phase by ± 2 % of methanol, flow rate by ± 0.2 ml/min and column temperature by ± 2 °C was performed. |
| |
| System-Suitability |
| |
| System suitability of the method was evaluated by analyzing the symmetry of the standard peaks, theoretical plates of the column. For this purpose five consecutive replicate analysis of the drug were assessed in order to investigate the suitability parameters including repeatability, peaks symmetry, and column efficiency (theoretical plates). |
| |
| Results and Discussion |
| |
| The HPLC method development for the determination of drugs has received a substantial consideration in the new era of technology, because of their importance in the quality control of drugs and drug products. The major purpose of developing this LC method was to attain determination of the drug in different pharmaceutical formulations under economical conditions that are applicable for routine quality control and research & development laboratories. |
| |
| A number of methods are available for MKT determination [8- 18], but many of them are used for certain specific purposes and no one can be generalized for MKT determination in its different forms of pharmaceutical dosages. The literature survey also revealed that almost all the methods developed so far have utilized acetonitrile as a major component in mobile phases [20], due to the supreme solubilizing properties and UV absorbance characteristics of acetonitrile, and there is no counterpart substitute for acetonitrile in the reverse-phase HPLC, UV application. However, keeping in view the increasing shortage of acetonitrile “Great Acetonitrile Shortage”, and high cost, laboratories are in search of cost-effective solutions to manage the impact on their research and business timelines. Also considering the chromatography type and the detection wavelengths in use, it may be possible to replace acetonitrile with methanol or with a longer chain alcohol. Also as Methanol is less expensive than acetonitrile and TFA or TCA etc., therefore the use of methanol as an alternative solvent to acetonitrile was evaluated in MKT analysis on large industrial basis, and a very simple and easy to use method has been developed. |
| |
| Validation of Method |
| |
| Linearity |
| |
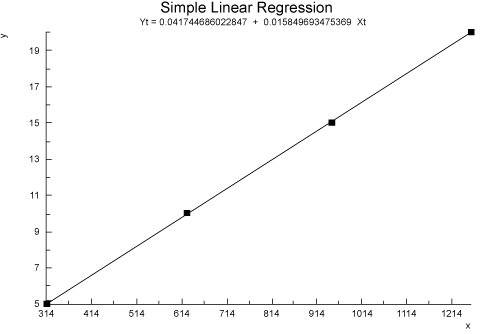
| The linearity ranges were found in the range of 5-20 μg/ml. The assay was judged to be linear as the correlation coefficient was greater than 0.995 by the least-square method. A linear correlation was found between the peak areas and the concentrations of Montelukast, in the assayed range. The regression analysis data are presented in Table 1 and Figure 2. |
| |
|
|
Figure 2: It shows Linearity of the method. |
|
| |
| Specificity |
| |
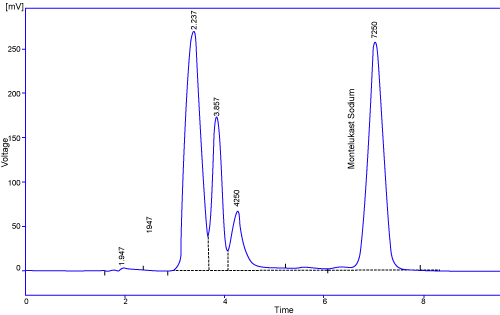
| Chromatogram shown in Figure 3, proves specificity or selectivity of the assayed method, as chromatogram of Montelukast in samples were found identical with standard chromatogram and no interference peak was observed in sample chromatogram, Peak purities higher than 98.0% were obtained in the chromatograms of sample solutions, demonstrating that other compounds did not co-elute with the main peaks (Figure 3). The chromatogram obtained with the mixture of the tablet excipients proves that here is no any interference from excipient and peak of interest fulfill all the requirements of symmetrical peak, and hence the specificity is confirmed. |
| |
|
|
Figure 3: It shows peaks of Montelukast in Suspension. |
|
| |
| Precision |
| |
| The precision of an analytical method is the degree of agreement among individual test results when the method is applied repeatedly to multiple sampling of homogeneous sample. Intra-day precision of the method was evaluated for montelukast at three different independent concentrations i.e. 8, 10, and 12 μg/ml (n=/3) by determining their assay. The RSD values ranged from 0.54 to 1.14% (Table 2) while Coefficient of variation (CV) of the assay results was NMT 3. Inter-day precision of the method was tested for 3 days at the same concentration levels. Solutions for calibration curves were prepared every day on freshly basis. Since the inter-day and intra-day precision obtained % RSD was <2.0 it indicates that the proposed method is quite precise and reproducible as shown in Table 2. |
| |
|
|
Table 2: Inter-Day and Intra-Day Precision of Montelukast (n = 3). |
|
| |
| Accuracy |
| |
| It was investigated by means of addition of Montelukast reference standards to a mixture of the tablet and suspension excipients. The mean recovery (n = 9) was 98.75% - 99.25% (RSD%=0.259) demonstrating the accuracy of the method. Recovery studies of the drug were carried out for the accuracy parameter at three different concentration levels i.e. multiple level recovery studies. A known amount of Montelukast standard was added into pre-analyzed sample and subjected to the proposed HPLC method. Percentage recovery was found to be within the limits (Table 3). |
| |
|
|
Table 3: Contents of Montelukast in the fixed dose combination. tablets and suspension |
|
| |
| Robustness |
| |
| The statistical analysis showed no significant difference between results obtained employing the analytical conditions established for the method and those obtained in the experiments in which variations of some parameters were introduced. The parameters used in system suitability test were asymmetry of the chromatographic peak, theoretical plates and capacity factor, as RSD of peak area for replicate injections. Thus, the method showed to be robust for changes in mobile phase methanol proportion, mobile phase pH, flow rate, and column temperature (Table 4). |
| |
|
|
Table 4: Robustness of the method. |
|
| |
| LOQ and LOD |
| |
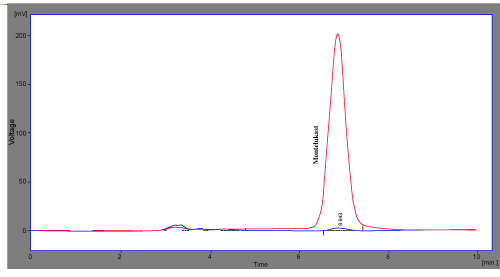
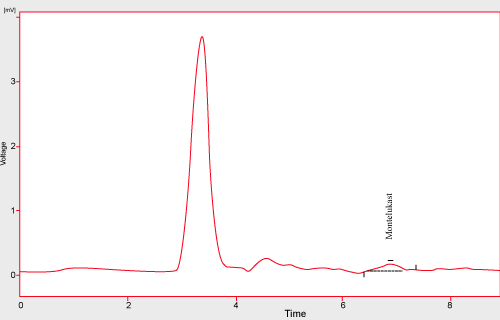
| The limit of detection was defined as the concentration for which a signal-to-noise ratio of 3 was obtained and, for quantitation limit; a signal-to-noise ratio of 10 was considered. According to this rule, Montelukast presented limits of detection of 1.25 ng /ml and limits of quantitation of 1.5 ng /ml. However, as the objective of the method is the quantitation of Montelukast, so that the values obtained should be considered as the limit of method sensitivity as well. Hence, the detection limit established was 1.25 ng /ml of Montelukast (Figure 5) and the quantitation limit was 1.5 ng /ml of Montelukast (Figure 4), the same compounds proportion was also found in the sample solutions injected onto the chromatograph. |
| |
|
|
Figure 4: It shows overlaid chromatogram of Montelukast in Tablet and its LOQ. |
|
| |
|
|
Figure 5: It shows Montelukast LOQ. |
|
| |
| System-Suitability |
| |
| System-suitability testing is an integral part of developing a chromatographic method and is used to verify that the resolution and reproducibility of the system are adequate for the analysis. Results are portrayed in Table 5, sharp and symmetrical peaks were obtained with good baseline. |
| |
|
|
Table 5: System Suitability Results. |
|
| |
| Conclusion |
| |
| A new simple, fast, precise and acetonitrile-free isocratic RP-HPLC method has been developed for the determination of Montelukast sodium in bulk, tablet and suspension dosage form. The developed method was found to be simple having short run time and economical. The results of the study indicate that the proposed HPLC method is precise, accurate and less time consuming. |
| |
| Acknowledgement |
| |
| The authors are very thankful to AGP Pvt. Ltd (Pakistan) for providing gift sample of Montelukast Sodium. |
| |
| |
| References |
| |
- Budavari S (1996) The Merck Index. 12th edition, Chapman & Hall, New Jersey, 1070, 163.
- Sweetman SC (2002) Martindale: The Complete Drug Reference. 33rd edition, Pharmaceutical Press, London 768.
- Goodman LS, Hardman JG, Limbird LE, Gilman AG (2001) Goodman and Gilman's the pharmacological basis of therapeutics. In: Hardman JG and Limbird LE, 10th edition, McGraw-Hill, New York, 677.
- Claesson HE, Dahlén SE (1999) Asthma and leukotrienes: antileukotrienes as novel anti-asthmatic drugs. J Intern Med 245: 205-227.
- Riccioni G, Vecchia RD, D'Orazio N, Sensi S, Guagnano MT (2003) Comparison of montelukast and budesonide on bronchial reactivity in subjects with mild-moderate persistent asthma. Pulm Pharmacol Ther 16: 111-114.
- Simons FE, Villa JR, Lee BW, Teper AM, Lyttle B, et al. (2001) Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J Pediatr 138: 694-698.
- Valacer DJ (1999) New treatments for asthma: the role of leukotriene modifier agents. J Natl Med Assoc 91: 26S-39S.
- Radhakrishna T, Narasaraju A, Ramakrishna M, Satyanarayana A (2003) Simultaneous determination of montelukast and loratadine by HPLC and derivative spectrophotometric methods. J Pharm Biomed Anal 31: 359-368.
- Alsarra IA (2004) Development of a stability-indicating HPLC method for the determination of montelukast in tablets and human plasma and its applications to pharmacokinetic and stability studies. J Saudi pharm 12: 136-143.
- Amin RD, Cheng H, Rogers JD (1995) Determination of MK-0476 in human plasma by liquid chromatography. J Pharm Biomed Anal 13: 155-158.
- Liu L, Cheng H, Zhao JJ, Rogers JD (1997) Determination of montelukast (MK-0476) and its S-enantiomer in human plasma by stereoselective high-performance liquid chromatography with column-switching. J Pharm Biomed Anal 15: 631-638.
- Ochiai H, Uchiyama N, Takano T, Hara K, Kamei T (1998) Determination of montelukast sodium in human plasma by column-switching high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl 713: 409-414.
- Kitchen CJ, Wang AQ, Musson DG, Yang AY, Fisher AL (2003) A semi-automated 96-well protein precipitation method for the determination of montelukast in human plasma using high performance liquid chromatography/fluorescence detection. J Pharm Biomed Anal 31: 647-654.
- Smith GA, Rawls CM, Kunka RL (2004) An automated method for the determination of montelukast in human plasma using dual-column HPLC analysis and peak height summation of the parent compound and its photodegradation product. Pharm Res 21: 1539-1544.
- Hoang TH, Farkas R, Wells C, McClintock S, Di Maso M (2002) Application of pressurized liquid extraction technology to pharmaceutical solid dosage form analysis. J Chromatogr A 968: 257-261.
- Saravanan M, Siva Kumari K, Pratap Reddy P, Naidu MN, Moses Babu J, et al. (2008) Identification, synthesis, isolation and spectral characterization of potential impurities of montelukast sodium. J Pharm Biomed Anal 48: 708-715.
- Papp R, Luk P, Mullett WM, Kwong E (2007) A rapid and sensitive method for the quantitation of montelukast in sheep plasma using liquid chromatography/tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 858: 282-286.
- Al Omari MM, Zoubi RM, Hasan EI, Khader TZ, Badwan AA (2007) Effect of light and heat on the stability of montelukast in solution and in its solid state. J Pharm Biomed Anal 45: 465-471.
- Chauhan B, Shubha Rani, Nivsarkar M, Padh H (2006) New liquid liquid extraction method for determination for montelukast in small volume human plasma samples using HPLC with fluorescence detector.Indian J Pharm Sci68: 517-520.
- Zhang XK, Elbin CS, Chuang WL, Cooper SK, Marashio CA, et al. (2008) Multiplex enzyme assay screening of dried blood spots for lysosomal storage disorders by using tandem mass spectrometry. Clin Chem 54: 1725-1728.
- International Conference on Harmonisation (1996) Guidance for Industry-Q2B Validation on Analytical Procedures: Methodology.U.S. Department of Health and Human Services Food and Drug Administration, CDER, CBER 1-10.
- United States Pharmacopeial Convention (2009) United State Pharmacopeia-National Formulary.
|
| |
| |