| Research Article |
Open Access |
|
| Maria G Bernardi1, Lyn Wright2 and Arun Aggarwal3* |
| 1Medical Student, University of New South Wales Rural Clinical School, Coffs Harbour, Australia |
| 2Registered Nurse, Department of Aged Care, Coffs Harbour Health Campus, Coffs Harbour, Australia |
| 3Associate Professor, Department of Neurology, Concord Hospital, Sydney, Australia |
| *Corresponding authors: |
Arun Aggarwal
Associate Professor
Department of Neurology
Concord Hospital, Sydney, Australia 2139
Tel: +61 2 9515 9815
Fax: +61 2 9817 6633
E-Mail: arun.a@sydney.edu.au |
|
| |
| Received January 24, 2011; Published August 27, 2012 |
| |
| Citation: Bernardi MG, Wright L, Aggarwal A (2012) Mild Cognitive Impairment is Under-Recognised in Newly Referred Patients with Parkinson’s Disease: Mini Mental State Examination (MMSE) Versus Montreal Cognitive Assessment (Moca). 1:280. doi:10.4172/scientificreports.280 |
| |
| Copyright: © 2012 Bernardi MG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Background: There is a high prevalence of Mild Cognitive Impairment (MCI) in patients with Parkinson’s disease, which has an impact on treatment, management, quality of life and prognosis. There remains a need for a brief, accurate and an easy to perform screening test, to reliably identify cognitive impairment. The nature of our study was to identify such a screening tool for use in clinical practice. The Mini Mental State Examination (MMSE) is currently the most commonly used screening tool in clinical practice, however, the Montreal Cognitive Assessment (MoCA) is considered to be a more sensitive measure of cognitive impairment in patients with Parkinson’s disease, as cognitive impairment in this group primarily involves executive function, attention, verbal fluency and visuo-spatial skills, which are not assessed adequately by the MMSE. |
| |
| Method: A sample of 46 participants, 35 newly referred patients with Parkinson’s disease and 11 population controls were recruited from a monthly specialist outreach Parkinson’s disease clinic in a regional centre of Australia, over a 6 month period. Information was obtained regarding age, ethnicity, years of schooling and awareness of cognitive impairment and reduced mood. MMSE and MoCA were performed on all participants as part of their initial multi-disciplinary assessment. |
| |
| Results: Participants with Parkinson's disease had significantly lower MoCA scores 23.2 ± 4.8 than MMSE scores 27.2 ± 2.6, p=<001. In participants with Parkinson's disease, the MMSE identified cognitive impairment (score of < 27) in 7 (20.0%), while the MoCA identified a further 13 (37.1%) (score of <26), indicating 57.1% of patients had a degree of cognitive impairment, that was unrecognised. This is even present in the early stages of the disease as the average number of months since diagnosis in our study was only 12 months and 68.6% of our participants had “mild” Parkinson’s disease with their Hoehn & Yahr stage being 2 or less. The MoCA test however took slightly longer to perform 9.4 ± 3.2 minutes compared to MMSE duration 7.6 ± 2.2 minutes, p=0.01. |
| |
| Conclusions: Our study was performed on newly referred patients to a Parkinson’s disease clinic, with a majority being at the early stages of Parkinson’s disease and many not having a formal diagnosis of Parkinson’s disease until they attend the clinic. The study demonstrates a clear difference in MoCA and MMSE sensitivity in an Australian population. There was a lack of recognition on the part of referring clinicians and patients themselves of the presence of cognitive impairment. It also provides additional support for the use of the MoCA as a screening tool in clinical practice on patients with Parkinson’s disease. Even though the MoCA takes slightly longer to perform, the increased sensitivity justifies its use in clinical practice. |
| |
| Keywords |
| |
| Parkinson’s disease; Neuropsychological tests; Cognition disorders; Screening |
| |
| Introduction |
| |
| Dementia and cognitive impairment are common in patients with Parkinson's disease [1] with the prevalence of Mild Cognitive Impairment (MCI) in Parkinson’s disease reported to being around 21% [2] and dementia as high as 80% [3]. This has a negative impact on prognosis and quality of life. Early identification of cognitive impairment may influence drug treatment and allow appropriate support to patients and their careers. Neuropsychological testing is considered to be the gold standard for assessing cognitive impairment but this assessment is time consuming and requires trained assessors [4]. |
| |
| The most commonly used instrument in busy clinical consultations is the brief Mini-Mental Status Examination (MMSE) [5]. A MMSE score of < 27 is considered to indicate MCI and ≤ 24 to indicate the presence of dementia [6]. Recent studies recommend a slightly higher score of < 24 be used to indicate the presence of dementia [7]. |
| |
| The MMSE has been found to be insensitive to mild forms of cognitive impairment in patients with Parkinson’s disease [8-9], as it primarily assesses memory and language skills, whereas the areas of cognitive impairment in patients with Parkinson’s disease primarily involve executive function, attention, verbal fluency and visuo-spatial skills [10]. |
| |
| As a result, two Parkinson’s disease specific cognitive scales, Parkinson Neuropsychometric Dementia Assessment (PANDA) [11] and Scales for Outcomes in Parkinson disease-Cognition (SCOPACog) [12] were designed. However, they too are limited by the time required to perform them and the areas of cognitive function they assess. Subsequently, there was still a need for a brief, accurate and easily performed screening test. |
| |
| The Montreal Cognitive Assessment (MoCA) [13] has been designed to address some of these limitations and has been found to have high sensitivity and specificity for mild cognitive impairment [14]. In addition, the MoCA has been shown to have adequate psychometric properties to allow it to be used as a screening instrument for the detection of mild cognitive impairment in Parkinson’s disease, as it has been shown to correlated highly with a neuropsychological battery (r=0.72) [15]. |
| |
| A Task Force review of five potential scales of global cognition (MMSE and MoCA, and three PD-specific instruments, the Mini- Mental Parkinson (MMP), PANDA and SCOPA-Cog has recommended that the MoCA is the most suitable measure for screening for mild cognitive impairment and dementia in clinical trials of Parkinson's disease [16]. In another study, MoCA was compared to SCOPA-Cog and MMSE and found to be a suitably accurate test that can be used for screening all levels of cognition in PD. A MoCA score < 26 is considered to indicate mild cognitive impairment and < 21 to indicate the presence of dementia [17]. |
| |
| The aim of this study was to compare the MMSE and MoCA cognitive screening tools in newly referred patients with Parkinson’s disease in an Australian population, which is yet to be reported, to identify whether cognitive impairment is under-recognised in this group of patients. |
| |
| Methods |
| |
| A prospective study was performed in a regional specialist outreach Parkinson’s disease clinic in Australia, held monthly over a 6-month period. Ethics approval for this study was granted by the Mid North Coast Human Research Ethics Committee. Written consent was gained from all participants. Participants consisted of newly referred patients to the Parkinson’s disease clinic and their accompanying partners being used as controls. Patients who were non-English speaking or severe aphasia were excluded. |
| |
| Each participant was assessed with the MoCA and MMSE by one of two investigators (LW or MB). There was a ten minute break between assessments. The scores were recorded for all 46 participants. The first 29 participants (18 patients and 11 controls) also had the time taken to perform each test recorded. Participant age, sex, level of schooling, history of subjective cognitive impairment, history of subjective mood changes and current usage of antidepressant or anti-anxiety medication were recorded. After the initial assessment, information regarding the patients Parkinson’s Disease status was recorded, including Hoehn & Yahr stage, duration of symptoms and medication usage. The diagnostic criteria proposed by the Queen Square Brain Bank was used to diagnose Parkinson’s disease [18]. |
| |
| Statistical analysis was performed using PASW 18.0.2 for Windows. The MMSE cut off score for mild cognitive impairment of < 27 was considered to indicate MCI and ≤ 24 to indicate the presence of dementia [6]. A MoCA score < 26 was considered to indicate mild cognitive impairment and < 24 to indicate the presence of dementia [13]. Outcome measures were the MMSE and MoCA scores and time taken to perform each test. Significance levels were set to 5% and all stated significance values are exact 2-tailed. 95% confidence intervals were estimated as a measure of uncertainty of effect. Differences between the test scores and time taken were analysed using paired student t-test. Between group differences were also analysed using an independent student t-test. |
| |
| Results |
| |
| A total of 46 participants, 35 newly referred Parkinson’s disease patients and 11 controls were recruited. No participants were excluded and no participants were aware of cognitive impairment prior to their assessment. 4 participants had a previous diagnosis of depression, 3 Parkinson’s disease patients and 1 control. Patient and control results are summarised in Table 1. |
| |
|
|
Table 1: Demographic and clinical characteristics of subjects. |
|
| |
| The Parkinson’s disease patient group was slightly older, 71.2 compared to 65.0 years of age, but this was not statistically significant (p=0.06). The number of years of schooling was similar in both groups, 10.7 years in patients compared to 10.2 years in the control group. |
| |
| Our study was performed on newly referred patients, and not surprisingly, the majority of our participants, 68.6% had only mild Parkinson’s disease with Hoehn & Yahr stage 2 or less. The average number of months since the diagnosis of Parkinson’s disease was only 12 months. The majority of our participants had their diagnosis confirmed when they attended the clinic. The average dose of levodopa in our group was low at the time of recruitment, 450 mg per day and there were no participants on a dopamine agonist. |
| |
| |
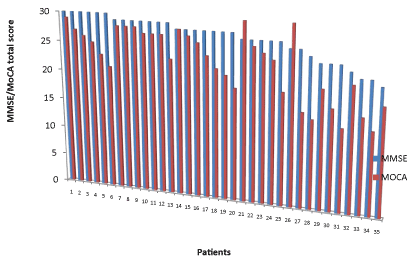
| The Parkinson’s disease patient group had a lower average MoCA score than the control group, 23.2 ± 4.8 compared to 26.7 2.7 (mean difference 4.0, 95% CI 2.8-5.2, t = 6.5782, p=<0.03), and more importantly, none of these patients were aware of having cognitive impairment prior to their assessment. Controls also had lower MoCA scores (26.727) than MMSE scores (28.4±1.6) (mean difference 1.6, 95% CI 0.3-3.0, t = 2.6312, p=0.03). This is demonstrated graphically in Figure 1. |
| |
|
|
Figure 1: Graphical comparison of MMSE and MoCA scores in participants with Parkinson's disease. |
|
| |
| Test duration was also significantly different with both groups. MoCA took longer to perform in participants with Parkinson's disease (9.4 ± 3.2 minutes) compared to MMSE (7.6 ± 2.2 minutes) (mean difference -1.9, 95% CI -3.2- -0.1, t=3.0977, p=0.01). Similarly, MoCA took longer to perform in controls, (8.3 ± 2.4 minutes) compared to MMSE (6.1 ± 1.7 minutes) (mean difference -2.3, 95% CI -3.6- -1.0, t=3.8326, p=0.01). |
| |
| In the patient group, MMSE identified cognitive impairment in seven patients (20.0%) while the MoCA identified a further thirteen (37.1%), indicating 57.1% of patients had mild cognitive impairment. The discrepancy between MMSE and MoCA in the participants who did not have cognitive impairment on MMSE was large. For example, three participants scored a perfect 30/30 on MMSE, but scored 21, 23 and 25 on MoCA. This observation was supported by a significantly larger mean difference in scores for these thirteen participants than the mean overall difference, 6.6 ± 2.6 versus 4.0 ± 3.6, (95% CI 0.4-4.8, t=2.3925, p=0.02). |
| |
| Discussion |
| |
| This study was performed in a monthly outreach specialist Parkinson’s disease outpatient clinic in regional Australia, which has limited specialist availability. In a regional setting, it is vital for cognitive impairment to be able to be screened promptly and reliably, using a tool that requires minimal training. Even though neuropsychological testing is considered to be the gold standard for assessing cognitive impairment, this assessment is not readily accessible in a regional setting and is too time consuming to be done routinely in a busy clinical practice. |
| |
| This study was performed to compare the MMSE and MoCA cognitive screening tools in Parkinson's disease in an Australian population. We found that the MoCA detected more cases of cognitive impairment in patients than MMSE, supporting the findings of a number of previous studies [10,14-15]. This study also supports the findings of the Task Force recommending MoCA as a minimum cognitive screening measure in Parkinson’s disease [16]. |
| |
| These results highlight two important points. Firstly, MoCA is comparable to MMSE in assessing cognitive impairment. In all cases of cognitive impairment on MMSE, MoCA was also abnormal. In addition, MoCA detected additional cases of mild cognitive impairment, when the MMSE was “normal”. We accept that some readers may still consider that detailed neuropsychological assessments are still needed to validate these results, but previous studies have shown that MoCA has adequate psychometric properties to allow it to detect mild cognitive impairment in Parkinson’s disease and it highly correlates with a neuropsychological battery (r=0.72) [15]. The aim of our study was to identify a screening tool for use in clinical practice that was brief and easy to administer in a busy clinical practice. |
| |
| Secondly, our study reinforces the value of screening for Parkinson’s disease patients for cognitive impairment early in the disease process. Of our newly referred patients, twenty (57.1%) were found to have mild cognitive impairment. This was present in patients with mild Parkinson’s disease and patients early in their disease process, as the average dose of levodopa in our patient group was relatively low at 450 mg per day and no participants were on a dopamine agonist. |
| |
| This implies a lack of subjective recognition on the part of referring clinicians and patients themselves regarding the presence of cognitive impairment. Lack of recognition of cognitive impairment may result in prescription of inappropriate drug therapies such as dopamine agonists and anti-cholinergics, which may exacerbate cognitive problems. Our study has a higher prevalence than previously documented [1], despite the relatively young average age of our participants, which supports our hypothesis that cognitive impairment in Parkinson’s disease is under-recognised in the community and also supports the need for an appropriate brief screening test. |
| |
| Depression is common and unrecognised in the early stages of Parkinson’s disease and can impact on cognitive function [19]. In our study, we did not use a specific depression rating scale and therefore may form limitation in our study. We relied on self-reporting and antidepressant therapy as indicating the presence of depression. 4 of our participants had a previous history of depression, 3 Parkinson’s disease patients and 1 control. |
| |
| Cognitive impairment not only impacts on patients with Parkinson’s disease and their careers, but also the community when the economic costs of nursing home and hospital care are recognised. An additional benefit to the community is that mild cognitive impairment predicts future cognitive decline [10]. A sensitive screening tool for detecting cognitive impairment may provide an opportunity to provide patients with appropriate early support and pharmacological intervention. Neuropsychological testing is not appropriate for this purpose as it is time consuming and requires trained assessors. |
| |
| This study provides additional support for the use of the MoCA as a screening tool in clinical assessment of patients with Parkinson’s disease and demonstrates a clear difference in MoCA and MMSE sensitivity in an Australian population of patients with Parkinson’s disease. This study identifies the need for a more comprehensive validation study in the wider Australian population. |
| |
| Acknowledgements |
| |
| We would like to acknowledge the assistance of Cathie MacKay for her coordination of participants, Peter Spence and the Mid-North Coast Division of General Practice (MNCDGP) for their support for our study. |
| |
| Financial Disclosures |
| |
| All authors declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; any other relationships or activities that could appear to have influenced the submitted work. |
| |
| |
| References |
| |
- Marder K (2010) Cognitive impairment and dementia in Parkinson's disease. Mov Disord 25: S110-S116.
- Caviness JN, Driver-Duckley E, Connor DJ, Sabbaghh MN, Hentz JG, et al. (2007) Defining mild cognitive impairment in Parkinson’s disease. Mov Disord 22: 1272-1277.
- Aarsland D, Kurz MW (2010) The epidemiology of dementia associated with Parkinson’s disease. J Neurol Sci 289:18-22.
- Zadikoff C, Fox SH, Tang-Wai DF, Thomsen T, de Bie RM, et al. (2008) A comparison of the Mini Mental State Exam to the Montreal Cognitive Assessment in identifying cognitive deficits in Parkinson's Disease. Mov Disord 23: 297-299.
- Folstein MF, Foltein SE, McHugh PR (1975) “Mini-Mental State”: A Practical Method for Grading the Cognitive State of Patients for the Clinican. J Psychiatr Res 12: 189-198.
- Rosenthal E, Brennan L, Xie S, Hurtig H, Milber J, et al. (2010) Association between cognition and function in patients with Parkinson's disease with and without dementia. Mov Disord 25: 1170-1176
- Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, et al. (2007) Diagnostic procedures for Parkinson’s disease dementia: Recommendations from the Movement Disoder Society Taskforce. Mov Disord 22: 2314-2324.
- Nazem S, Siderowf AD, Duda JE, Have TT, Colcher A, et al. (2009) Montreal Cognitive Assessment performance in patients with Parkinson's Disease with "normal" global cognition according to Mini-Mental State Examination score. J Am Geriatr Soc 57: 304-308.
- Riedel O, Klotsche J, Spottke A, Deuschl G, Förstl H, et al. (2008) Cognitive impairment in 873 patients with idiopathic Parkinson’s disease: Results from the German Study on Epidemiology of Parkinson’s Disease with Dementia (GEPAD). J Neurol 255: 255-264.
- Mamikonyan E, Moberg PJ, Siderowf A, Duda JE, Have TT, et al. (2009) Mild cognitive impairment is common in Parkinson's disease patients with normal Mini-Mental State Examination (MMSE) scores. Parkinsonism Rel Disord 15: 226-231.
- Kalbe E, Calabrese P, Kohn N, Hilker R, Riedel O, et al. (2008) Screening for cognitive deficits in Parkinson’s disease with the Parkinson Neuropsychometric Dementia Assessment (PANDA) instrument. Parkinsonism Relat Disord 14: 93-101.
- Marinus J, Visser M, Verwey NA, Verhey FR, Middelkoop HA, et al. (2003) Assessment of cognition in Parkinson’s disease. Neurologu 61: 1222-1228.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, et al. (2005) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695-699.
- Gill DJ, Freshman A, Blender JA, Ravina B (2008) The Montreal Cognitive Assessment as a screening tool for cognitive impairment in Parkinson's disease. Mov Disord 23: 1043-1046.
- Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, et al. (2009) Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson's disease. Neurology 73: 1738-1745.
- Chou KL, Amick MM, Brandt J, Camicioli R, Frei K, et al. (2010) A recommended scale for cognitive screening in clinical trials of Parkinson’s disease. Mov Disord 25: 2501-2507.
- Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, et al. (2010) The MoCA: Well suited screen for Cognitive Impairment in Parkinson Disease. Neurology 75: 1717-1725.
- Hughes AJ, Daniel SE, Blankson S, Lees AJ (1993) A clinicopathologic study of 100 cases of Parkinson’s disease. Arch Neurol 50: 140-148.
- Bhidayasiri R, Trung DD (2012) Therapeutic strategies for nonmotor symptoms in early Parkinson’s disese: the case for a higher priority and stronger evidence. Parkinsonism Rlate Disord 18: S110-113.
|
| |
| |