| Research Article |
Open Access |
|
| D. Akkinepally1, A. Arif2, E. Gaffoor1, L.Krishna2, Mahendranadh R1 and S. Dravida1* |
| 1Tran-Scell Biologics Pvt Ltd. Jubilee Hills, Hyderabad, AP, India |
| 2Sri Sai Dental College, Vikarabad, A.P, India |
| *Corresponding authors: |
S. Dravida
Tran-Scell Biologics Pvt Ltd. Jubilee Hills, Hyderabad, AP, India
Tel: 040-23549696/9797/9898
Fax: 040-23549393
E-mail: suba.dravida@tran-scell.com |
|
| |
| Received August 28, 2012; Published September 01, 2012 |
| |
| Citation:Akkinepally D, Arif A, Gaffoor E, Krishna L, Mahendranadh R, et al. (2012) Laser Irradiation on Human Umbilical Cord Stroma Derived Mesenchymal Stem Cells for Regenerative Medicine Applications in vitro Study. 1:290. doi:10.4172/scientificreports.290 |
| |
| Copyright: © 2012 Akkinepally D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Mesenchymal Stem Cells (MSCs) were exposed to single dose of laser irradiation and cellular properties were assessed by Trypan Blue Method, MTT assay, surface marker analysis, LDH secretion and genomic integrity assays. This investigation revealed that the stem cell culture characteristics were retained post irradiation with documented increase in the yield of cultured cells in less time. The authors speculate that the laser irradiation on cultured clinical grade cells aid in packaging these cells for regenerative therapeutic applications.` |
| |
| Keywords |
| |
| Chromosomal stability; Clinical grade; Low level laser irradiation; Mesenchymal stem cells; Proliferation; Umbilical cord tissue |
| |
| Abbrevations |
| |
| hUCT: human Umbilical Cord Tissue; MSCs: Mesenchymal Stem Cells; DMEM: Dulbecco Modified Eagle’s Medium; FBS: Fetal Bovine Serum; PBS: Phosphate Buffer Saline; EGF: Epidermal Growth Factor; VEGF: Vascular Endothelial Growth Factor; LDH: Lactate Dehydrogenase; PI: Propidium Iodide; GAA: Glacial Acetic Acid |
| |
| Introduction |
| |
| Mesenchymal Stem Cells (MSCs) have self renewal and multi lineage differentiation potential [1,2]. It was reported that these MSCs differentiate into osteocytes, chondrocytes, adipocytes, astrocytes, myocytes under respective culture environment [3], thus gained a promising role in regenerative medicine. In addition, MSCs act as stromal support for the haematopoetic stem cells in bone marrow [4]. MSCs can be isolated from adult peripheral blood [5], bone marrow [1], adipose tissue, deciduous tooth, trabecular bone [6], umbilical cord blood [7,8], skeletal muscle, adipose [9], amniotic fluid [10]. Clinical data on immunosuppressive role of bone marrow derived MSCs is latest to the published literature with hematopoietic stem cells transplantations. Though bone marrow represents the major source, the aspiration involves invasive procedure, low frequency and count of viable BM- MSCs with age [5], bleeding, infection and chronic pain at the site of aspiration. Umbilical Cord Blood and Tissue form the alternate sources with no ethical issues, easy accessibility, no pain, no risk factors involved to donors in aseptic collection minimizing viral contamination [8]. The yield from umbilical cord blood is low compared to human Umbilical Cord Tissue (hUCT) although both the sources are ontogenetically less exposed to the immune system [11]. MSCs are regarded as therapeutic tools in cell therapy, tissue repair in degenerative or damaged locations and in allogenic transplantations. |
| |
| Standard culture conditions include fetal bovine serum (FBS) in the growth medium and MSCs are harvested after enzymatic digestion from the extracellular content of cord tissue and seeded for cellular growth and proliferation. Minimum couple of million cells can be harvested in a month’s growth period in the tissue culture ware. One of the cell therapy challenges is the availability of the right number of cells for infusion in regenerative applications. |
| |
| To propose hUCT as a promising source for therapeutic cells, we postulate that obtaining sufficient cell numbers of high quality in the shortest period would be the required property in regenerative transplant medicine and reconstructive surgery. Factors inducing or affecting the proliferation of cells are usually chemical in composition. |
| |
| Laser is a monochromatic, coherent and directional energy which has both stimulatory and inhibitory effects on cells, with a major role in wound healing, bone adhesion, immunological and clinical aspects [12]. Low Level Laser Intensity (LLLI) of wavelength 600-810 nm was found to show positive biostimulatory effect on MSCs by enhancing the cellular ATPases activity and hence the proliferative capacity. Red to near infrared region of Electro Magnetic Radiation was shown to be absorbed easily by mitochondria of cells, elevating the respiratory chain components, resulting in the increase of reactive oxygen species (ROS), and adenosine triphosphate (ATP)/or cyclic AMP, and initiating a signaling cascade which promotes cellular proliferation and cytoprotection [13]. |
| |
| In this present study, we evaluated the effect of LLLI of 940 nm wavelength on the clinical grade MSCs cultured from hUCT to estimate the proliferation and other cellular kinetics including the chromosomal stability of cells. The objective of the current study was to assess the regenerative medicine potential of the clinical grade MSCs after LLLI exposure. |
| |
| Materials and Methods |
| |
| Isolation of hUCT- MSCs |
| |
| Human Umbilical Cord Tissue (hUCT) was collected after full term baby deliveries by Caesarean Section with the informed consent signed by the respective donors. In-house Ethics Committee clearance obtained for the research proposed. The collected tissue was transported to the lab aseptically at ambient temperature. One inch hUCT was then digested with Trypsin-EDTA (Gibco – Invitrogen, CA) solution for 1hr. |
| |
| The activity of digestion enzyme (Gibco - Invitrogen, CA) was ceased by addition of Dulbecco’s Modified Eagle’s Medium (DMEM/F12 + Glutamax – 1X) (Gibco- Invitrogen, CA) with 5% Fetal Bovine Serum (FBS) (Gibco - Invitrogen, Mexico). The digested tissue rinsed twice with Dulbecco’s Phosphate Buffer Saline 1X (DPBS) (Gibco, Invitrogen , NZ) and minced into small fragments of 1-2 mm3 and explanted in 60 mm2 petri- dishes (Corning, NY) with minimal amount of complete growth medium consisting of DMEM/F12 (Gibco- Invitrogen, CA) with 5% FBS ((Gibco - Invitrogen, Mexico) supplemented with 0.001% Recombinant human Endothelial Growth Factor (EGF) (Gibco - Invitrogen, CA) and 0.001% Vascular Endothelial Growth Factor (VEGF) (Gibco- Invitrogen, CA) and 0.002% Pencillin - Streptomycin (Pen strep) (Gibco- Invitrogen, CA) in humidified 37°C chamber with 5% CO2 for a week to initiate cells from the explants. The cell culture was maintained with the replacement of medium every 48 hours. |
| |
| Cell expansion |
| |
| Ten days later 70-80% confluent primary culture was trypsinised and replated to the next passage with the seeding density of 1 X 105 cells per each T-25 flask (Corning, NY). Non-adherent cells were removed and replenished with fresh complete growth medium after 48 hours. The cultures were incubated in humidified 37°C chamber with 5% CO2 for expansion. The medium was changed thrice a week, thereafter. |
| |
| Treatment procedure |
| |
| The flasks with semi confluent cells were divided into Control group and Test group. |
| |
| Control group: Cell culture flasks with no treatment. |
| |
| Test group: Cell culture flasks with LLLI treatment (irradiation). |
| |
| The medium in the flask was removed and replaced with 2mL of Dulbecco’s Phosphate Buffer Saline (DPBS) (Gibco – Invitrogen, NZ). Irradiation was delivered using GaAIAs semiconductor laser diode (ezlase, USA), mostly applied in dentistry of 940 nm wavelength with the maximum output power of 7.0 W. The cell cultures were irradiated with the following parameters: 4J/cm2 of energy density, total energy of 120 J, total power of 0.2 W at a distance of 2 cm in continuous pulse mode. Post treatment, the flasks were replenished with 3mL of complete growth medium discarding DPBS (Gibco- Invitrogen, NZ |
| |
| Phenotyping |
| |
| Microscopic evaluation: Morphological evaluation of the treated and control groups was recorded 24 hrs later under inverted phase contrast microscope (Motic AE31, Hong Kong). The phase contrast was coupled with Moticam 2500, 5.0 megapixels and Motic Images Plus 2.0 Multi-Language Software. The cell morphology of both the groups was observed for blebbing, shrinkage and nuclear fragmentation at thrice within 24 hrs post treatment 10X magnification. |
| |
| Flow cytometry analysis |
| |
| Cell Cycle Analysis: Both groups were stained with Propidium Iodide (PI) for the enumeration of cells in different phases of cell cycle. MSCs were harvested, centrifuged for 5 minutes, washed with cold PBS (pH 7.5), cells permeabilisation and fixing was performed by incubating overnight with 70% ethanol at 4°C. Then cells were washed with PBS and suspended in Propidium Iodide (PI) solution (PBS containing 50μg/mL PI) (Sigma-Aldrich, USA), 100 μg/mL RNAse (Himedia, India) at a cell density of 1 × 106, incubated at 40C in dark for 2hrs. Data acquisition and Cell cycle profile analysis were performed using CellQuest software on BD Calibur flow cytometer (BD Biosciences, USA). |
| |
| Surface Marker Analysis: Surface Marker Analysis was performed by incubating the cells with respective primary antibodies for CD 90, CD105 and CD45 and analyzed on Beckman Coulter FC- 500 series. |
| |
| Proliferation – evaluation: |
| |
| Cell counting and viability |
| |
| Cell Counting and Viability of both the groups were performed using Trypan Blue Method (Gibco - Invitrogen, CA) 48 hrs post irradiation. Trypan Blue is the dye used for identification of viable cells in a specific population, where the chromophore, negatively charged, is absorbed only by the dead cells in the population where the membrane is permeable to the chromophore. As a result the live cells will be colorless and the dead cells stains up the blue color. The cell suspension was enumerated using Automated Cell Countess Machine (Invitrogen, USA) both for Control and Test groups. |
| |
| MTT Assay |
| |
| The mitochondrial activity was quantified in both the groups when the cells reached 70% confluence. It was measured using 3-(4,5-dimethylthiazol-2-yl)- 2,5- diphenyltetrazolium bromide (MTT) assay. The protocol was followed as per the kit manual instructions (Invitrogen, USA) and the OD 580nm was read spectrophotometrically. |
| |
| Colony Forming Units |
| |
| The frequency of colony forming was assessed using Grid Method under 4X magnification (Motic, USA), 48 hrs post irradiation. Aggregates containing more than fifty cells were enumerated as a colony. Such colonies in both groups were counted and an average count was determined. The colonies in each square of the grid were counted manually and an average number was derived using triplicates. |
| |
| Passaging- Rate& Capacity |
| |
| The cells post irradiation were continued for several passages and observed for population doubling time and documented in comparison to control groups. |
| |
| Biochemical analysis - spent medium |
| |
| Biochemical Analysis of the spent medium was performed 48 hours post treatment in both groups for glucose and Lactate dehydrogenase (LDH) using Hexokinase and IFCC method respectively. |
| |
| Chromosomal study |
| |
| Karyotyping: A Karyotype was generated for treated cells in comparison to control cells. The karyograms were analysed in both groups when the cultures reached full confluence, incubating in Colchemid. Old medium was aspirated out and fresh medium was added with 75μL of 0.01 % of Colchemid Solution (Himedia, India) and incubated for 2 hrs in humidified 5% CO2 incubator at 37°C. 8mL of Hypo solution was added to the cells discarding the medium containing colchemid and incubated for 30 min. The adherent cells were scrapped off using a cell scrapper and centrifuged twice for 10 min at 2500rpm and the pellet was fixed in ice cold Methanol: Glacial Acetic Acid (GAA). The hanging drop method was performed and cells were arranged for metaphase reading. |
| |
| Soft agar assay: The 35mm2 plates which were covered with a layer of 0.5% base agar in DMEM medium supplemented with 10% FBS and allowed to solidify. Tumor cell line C6o(rat glioma) (donated by Hyderabad Central University) was considered as the positive control. The internal control and test group cell cultures were trypsinized, gently mixed with 0.7% agar medium mixture and seeded on to the base agar plates in triplicate. After 2-3 weeks, the resistant colonies were stained with 0.005% crystal violet and observed under the microscope. Data were obtained from 3 independent experiments [14]. |
| |
| Statistical analysis |
| |
| Data is presented as mean of triplicate culture representation. |
| |
| Results |
| |
| MSC’s cultured from human cord tissue |
| |
| Cell growth initiation after 72 hrs of explants seeding was recorded. The primary cell culture was 90% confluence by next one week. Total time required for the cells to occupy 60mm2 petri-dish was 14 days. The cells after seeding into T-25 flasks reached almost 100% confluence in 7 days. |
| |
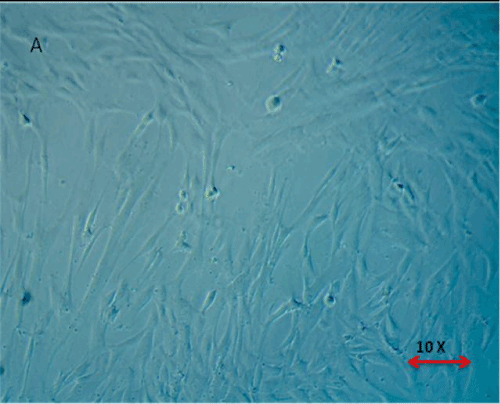
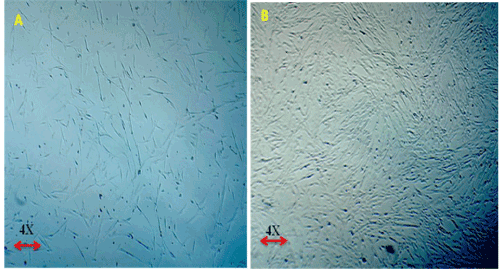
| Below findings represent that the cells were successfully isolated from hUCT and reached > 80% confluence in T-25 flasks in a week time. Morphologically, the cells isolated from hUCT appeared as a monolayer of adherent elongated structures with bipolar tapered ends within 24hrs of seeding under inverted phase contrast microscope of 4X magnification (Figure 1). There was no remarkable difference in both groups morphologically, but the cell density in Test group was observed to be intense compared to Control group (Figure 2), 48 hrs post irradiation. |
| |
| |
|
|
Figure 1: Morphological Appearance of Cells isolated from Umblical Cord Tissue (Huct) after 24 hrs. The Cells are found to be adherent monolayer with bipolar tapered and elongated fibroblast like cells. |
|
| |
|
|
Figure 2: (A,B) Proliferative Capacity of MSCs derived from human Umblical Cord Tissue (Huct). 2A- Control Group – Micrographs 2B – Test Group – Micrographs – 48 hrs post irradiation. Magnification 4X under phase contrast. The cells maintained their primary morphological characteristics in Test group (T) like normal Control post irradiation with intense proliferation in test group (T). |
|
| |
| Increased cell percentage in G- phase |
| |
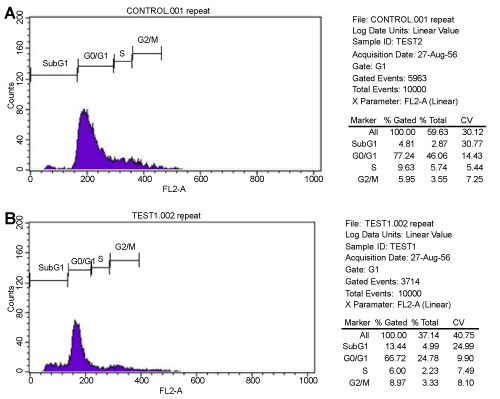
| The DNA content of MSCs in control and test groups was analyzed in FACS at OD 488 nm. The metaphase pattern was observed to be same in both groups. Majority of cells were found to be arrested in G0/G1 phase in the two groups. Necrotic cell death with G0/G1 arrest appeared in control cell group whereas apoptotic cell death with high incidence of cells in G0/G1 phase was recorded in test group. Additionally, more cells were enumerated at G2/M phase in test group compared to control (Figure 3) (Table 1). |
| |
|
|
Figure 3: (A.B) Cell Cycle distribution of Control and Test. Both groups were found to have G0/G1 arrest. The percentage of cells in G2/M phase were found to be more in Test group compared to control group. |
|
| |
|
|
Table 1: Cell cycle distribution using flow cytometer in both Control and Treated groups (%). |
|
| |
| Expressed surface marker profile |
| |
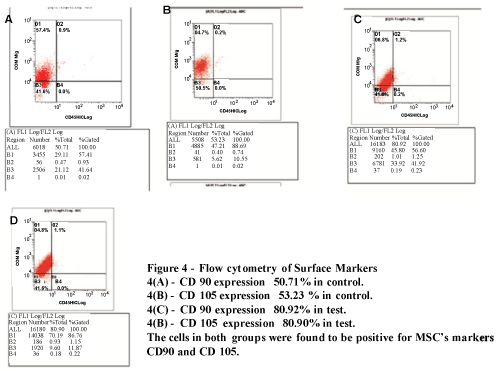
| The cells were found to be 80.92% positive for CD 90 and 80.90% for CD 105 and negative for CD 45 surface markers in test group whereas 50.71% of control cells expressed CD 90 and 53.23 % CD 105 (Figure 4A and 4B). |
| |
|
|
Figure 4: Flow cytometryof Surface Makers 4(A)-CD 90 expression 50.71% in control 4(B)-CD 105 expression 53.23% in control 4(C)-CD 90 expression 80.92 in control 4(D)-CD 105 expression 80.90% in control The cells in both groups were founf to be positive for MSC’s Markers CD 90 amd CD 105. |
|
| |
| Enhanced Stem Cell Kinetics Post Irradiation |
| |
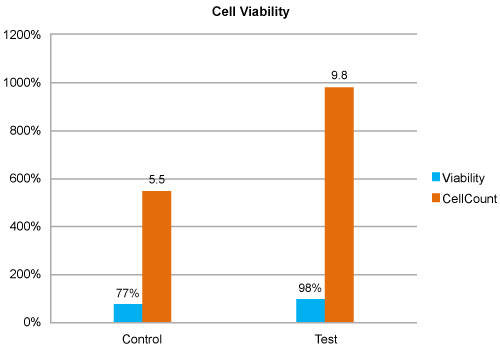
| Enhanced cell count and constant number of viable cells post treatment: MSCs showed 1.8 fold increase in cell count with constant viable cells in test group (9.8 X 105) compared to control (5.5 X 105), 48 hrs post irradiation (Figure 5). |
| |
|
|
Figure 5: Cell Viability Analysis. MSCs of Test group found to have maximum percentage of viable cells compared to control. |
|
| |
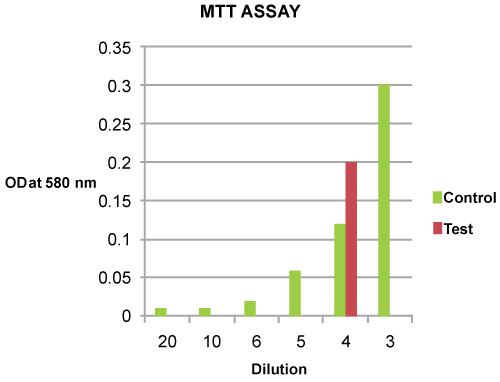
| Varied Mitochondrial Activity: The mitochondrial activity of the viable cells was detected to have increased twice by MTT assay at OD580 nm in test group (OD580 nm - 0.20) compared to control (OD580 nm - 0.10) (Figure 6). |
| |
|
|
Figure 6: MTT Analysis of Control and Test group. The Test OD was doubled (0.20) compared to control (0.10) with the cell density of 2.45 million cells. |
|
| |
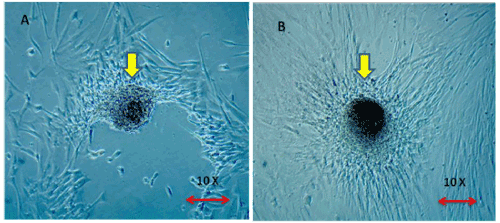
| Enriched colonies: The colony forming units of adhered monolayer MSCs were found to be dense and increased four- fold in test group (36 colonies) compared to control (9 colonies) (Figure 7A and 7B) (Table 2). |
| |
|
|
Figure 7: (A,B) Representative colony forming units in control and test 48 hrs post irradiation 7A. MSC colony captured in Control Group. 7B. MSC colony captured in Test Group. Magnification – 10x under Phase contrast Microscope. The Colony Formation Unit were found to be dense in the Test group compared to Control. |
|
| |
|
|
Table 2: Colony Forming Units - Count by Grid Method in control and test groups |
|
| |
| Decreased population doubling time: The doubling time in test group (104.8) was found to be reduced by 1.36 fold compared to Control group (76.85). |
| |
| Decreased glucose levels and increased LDH secretion into the medium |
| |
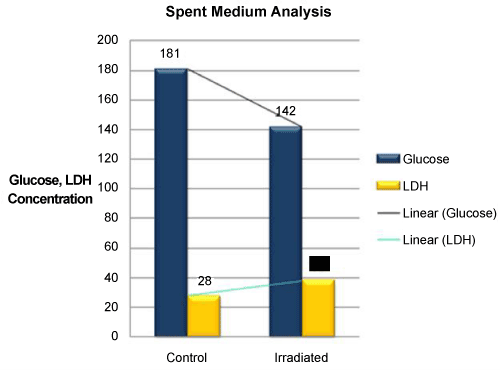
| An increment in the levels of glucose consumption (39%) and secretion of LDH (1/2 fold) into the medium was documented in Test group (Glucose - 142 mg/dL; LDH – 38 U/L) in comparison to control groups (Glucose - 181 mg/dL; LDH - 28 U/L) (Figure 8). |
| |
|
|
Figure 8: Comparision of Glucose consumption and LDH secretion into the medium in Control and Test cell culture groups. Decreased glucose levels and increase in LDH secretion into the medium was found in Test group 48 hours post irradiation. |
|
| |
| Chromosomal Study |
| |
| Normal Chromosomal Pattern: Karyograms of both the groups remained same without any specific induced mutational change owing to the laser exposure (Figure 9A and 9B). |
| |
|
|
Figure 9: [A,B] Karyograms 9 [A] – Metaphase of Control 9 [B] – Metaphase of Test Test group found to have same chromosomal pattern as control with no induced chromosomal aberrations. |
|
| |
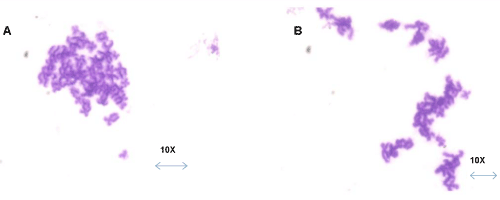
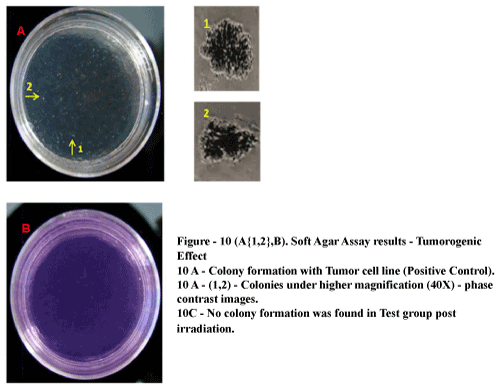
| No tumorogenic colonies observed post irradiation: The tumor derived cell line (positive control) developed sparse multicellular colony aggregates where as test group formed no such colony aggregates in soft agar (Figure 10A{1,2} and 10B). |
| |
|
|
Figure 10: Soft Ager Assay results – Tumorogenic Effect 10 A-Colony Formation with Tumor cell line (Positive Control). 10A(1,2)- Colonics under higher Magnification (40X)-phase contrast images. 10C-No colony information was found in test group post irradiation. |
|
| |
| Discussion |
| |
| The related literature and publications showed that low level laser therapy occupied a prominent role in biostimulation of cell cultures and hence implicated in tissue engineering, clinical applications like bone formation, immunomodulation, angiogenesis and wound healing. Abramovitch-Gottlib L, et al., proved that He-Ne laser in conjugation with coralline biomatrix system increased the cellular proliferation of BM- derived MSCs and differentiation into osteocytes and ex vivo ossification. Low level laser irradiation lead to excitation of electrons in electron transport chain, increase the ATP activity and thus in turn increase the proliferative capacity of cells [15]. In addition to the clinical promotion of healing the tissue lesions by LLL therapy, multiple in vitro studies of LLL on a different cell types such as fibroblasts, keratinocytes, lymphocytes stem cell harvested from bone marrow were published. The present study of exposing the cultured MSCs from hUCT was essentially undertaken to document the accelerated cell capacities retaining the type specific characteristics and thus for use in cellular therapies. In the series of experiments conducted in our study we have successfully harvested clinical grade MSCs from hUCTs and compared the proliferative and viability rates of the single dose irradiated cells to the control samples. |
| |
| Mihaela Pislea et al. [16]- documented in their study that major percentage of Jurkat T cells were found to be in Go/G1 Phase after low level laser irradiation. Our results implicated the repression of cells in S- phase and a slight increase in the apoptotic stage in irradiated samples compared to control samples. Mvula et al. [15], observed that the proliferative rate and percentage of viability increased in adipocyte derived stem cells lased with semiconductor diode at 636 nm wavelength and Wen-Tyng Li et al. [17], showed major increase of colonies of human bone marrow derived MSCs post irradiation. Xuejuan Gao et al. [13] stated that LLLI stimulates the mitochondrial activity in MSCs and thus increases the proliferation rate. Dominici et al. [18] concluded that MSCs derived from bone marrow found to be positive for CD 90 and CD 105 and found to be negative for CD 45 cell surface markers. In similar lines our observations allowed us to conclude significant enhancement of proliferative rate with the percentage increase in viability, colony formation of the MSCs from hUCT with no tumorogenic effect with single dose of LLL stimulation. Although there is a difference in the percentage of CD markers like CD 90 and 105 expressed in both the groups, we do not speculate the laser exposure to have a role in the variation of the expression pattern. The consumption of glucose was high in the irradiated cells compared to control ones directly indicated the accelerated stem cell proliferation specific to wavelength applied. The decreased population doubling time was found in irradiated group when compared to control group data. Here we report that the hUCT derived MSCs culture conserve the normal MSC like surface marker expression patterns along with the chromosomal stability after single dose light stimulation. |
| |
| Conclusion |
| |
| Despite the fact that above hypotheses have been published on several cell types, none discussed on retention of stem cell specific characterization profile along with the chromosomal stability post irradiation speculating an entirely different function of LLL wavelength used on hUCT derived MSCs. Our results show that the clinical utility of hUCT derived MSCs post irradiation with assessed cell capacities is not compromised. |
| |
| Acknowledgement |
| |
| The authors would like to thank Vitae Medical Center for clinical resources, Dr. Aleem. A. Khan, Mr. Sivaram Gunisetty, Prof. Prakash Babu (HCU), Mr. Anwar for the help provided to analyze the karyograms. |
| |
| |
| References |
| |
- Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, et al. (2005) Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 33: 1402-1416.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143-147.
- Halleux C, Sottile V, Gasser JA, Seuwen K (2001) Multi-lineage potential of human mesenchymal stem cells following clonal expansion. J Musculoskelet Neuronal Interact 2: 71-76.
- Barry FP, Murphy JM (2004) Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 36: 568-584.
- Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, et al. (2004) Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 103: 1669-1675.
- Purpura KA, Aubin JE, Zandstra PW (2004) Sustained in vitro expansion of bone progenitors is cell density dependent. Stem Cells 22: 39-50.
- Hua J, Qiu P, Zhu H, Cao H, Wang F, et al. (2011) Multipotent mesenchymal stem cells (MSCs) from human umbilical cord: Potential differentiation of germ cells. African Journal of Biochemistry Researc 5: 113-123.
- Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, et al. (2006) Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 91: 1017-1026.
- Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, et al. (2000) Adult Human Mesenchymal Stem Cell Differentiation to the Osteogenic or Adipogenic Lineage Is Regulated by mitogen-activated protein kinase. J Biol Chem 275: 9645-9652.
- Tsai MS, Lee JL, Chang YJ, Hwang SM (2004) Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod 19: 1450-1456.
- Secco M, Zucconi E, Vieira NM, Fogaca LL, Cerqueira A, et al. (2008) Multipotent stem cells from umbilical cord: cord is richer than blood. Stem Cells 26: 146-150.
- Walsh LJ, Glazewski JB, Mvula B (2008) Low Level Laser Therapy: 1, 2, 10.
- Gao X, Xing D (2009) Molecular mechanisms of cell proliferation induced by low power laser irradiation, J Biomed Sci 16: 4.
- Shuda M, Kwun HJ, Feng H, Chang Y, Moore PS (2011) Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J Clin Invest 121: 3623-3634.
- Mvula B, Moore TJ, Abrahamse H (2010) Effect of low-level laser irradiation and epidermal growth factor on adult human adipose-derived stem cells. Lasers Med Sci 25: 33-39.
- Pislea M, Seremet T, Katona G, Mocanu M, Doaga IO, et al. (2009) Low level long wavelength laser irradiation effects on cell cycle progression and apoptosis of energy restricted Jurkat t-cells. Romanian J Biophys 19: 1-18
- Wen-Tyng Li, Hui-Lin Chen, Cheng-Te Wang (2005) Effect of Light Emitting Diode Irradiation on Proliferation of Human Bone Marrow Mesenchymal Stem Cells. J Med Biol Eng 26: 35-42.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315-317.
|
| |
| |