|
|
| Sunil Wadgaonkar1, Natalia Grinkina1, Satish Gowda1, Kaumudi Somnay2 and Raj Wadgaonkar1* |
| 1SUNY Downstate and VA Medical Center, USA |
| 2Brooklyn and New York Hospital, Weill-Cornell Medical College, New York, USA |
| *Corresponding authors: |
Raj Wadgaonkar
Molecular Medicine Laboratory, Brooklyn and New York Hospital
Weill-Cornell Medical College, New York, USA
Tel: 718-270-226
E-mail: raj.wadgaonkar@downstate.edu |
|
| |
| Received November 09, 2012; Published August 30, 2012 |
| |
| Citation: Wadgaonkar S, Grinkina N, Gowda S, Somnay K, Wadgaonkar R (2012) Sphingosine Kinase Activation in Lipid Rafts and Pulmonary Endothelial Cell Survival. 1: 299. doi:10.4172/scientificreports.299 |
| |
| Copyright:© 2012 Wadgaonkar S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Signaling intermediates generated in plasma membrane lipid raft microdomains by Tumor necrosis factor-alpha (TNFα) binding to its receptor (TNFR), play a diverse role in downstream signaling. TNFα binding to the TNFR1 can elicit many different effector functions, including cellular apoptosis, cell survival signaling, JNK/p38 activation and NFκB activation. We have previously shown that in response to TNFα treatment, pulmonary artery endothelial cells (HPAEC) induce NFkB dependent survival signaling and are resistant to apoptotic cell death. To delineate pathways leading to survival in this study, we localized TNFR and associated binding proteins to the caveolin-expressing cellular fractions containing the lipid raft microdomains of the plasma membrane. We found that in response to TNFα treatment, sphingosine kinases (SphK1, SphK2) and TNFR proteins translocated to caveolin-enriched membrane lipid raft cellular fractions. Additionally, by immunoprecipitation we identified a time-dependent association of SphK1 with membrane Lyn kinase. This interaction was confirmed using reverse immunoprecipitation using Flag and Lyn specific antibodies. To understand if SphK1 uses Lyn kinase as an anchoring protein for membrane interactions, and whether this interaction has any functional role in the sphingolipid signaling pathway, we attenuated Lyn kinase expression by siRNA and studied the translocation-dependent SphK1 activation in the membrane lipid rafts. Our results suggested that the Lyn kinase-SphK1 interaction is involved in SphK1 activation, and this is required for protection against TNFα induced HPAE cell apoptosis. |
| |
| Introduction |
| |
| Human endothelial cells are resistant to apoptotic stimuli generated by TNFα, however, the signaling pathways which protect the endothelial monolayers from apoptosis are not clear. Several studies have shown that TNFα activates sphingomyelinases to convert sphingomyeline into ceramide, which has been found to be proapoptotic in in vivo as well as in vitro studies [1,2]. Ceramide and sphingosine usually inhibit proliferation and promote apoptosis, while the downstream metabolite S1P stimulates growth and suppresses ceramide-mediated apoptosis. Stimuli such as TNFα, interleukin-1β, or ionizing radiation degrade sphingomyelin, producing ceramide via action of sphingomyelinases [3,4]. In the pulmonary endothelium, this balance is essential for protection against lung injury. Ceramide, the central lipid second messenger generated in the sphingomyelin (SM) signal transduction pathway, is metabolized by ceramidase to yield sphingosine, which also exhibits its biological actions by modulating signaling enzymes and pathways [5].Sphingosine is further phosphorylated into S1P by the activation of sphingosine kinases [6-8]. Interestingly, the three lipid-mediators derived from SM hydrolysis exhibit contrasting biological actions. Ceramide regulates stress responses and apoptosis, while sphingosine modulates activity of both protein kinases and phosphatases as well as induces apoptosis. Both ceramide and sphingosine are implicated in linking cell surface receptors, such as TNFα and the Fas receptors, to apoptotic pathways. Conversely, S1P promotes growth, cell motility, and inhibits ceramidedependent apoptotic pathway. Although this pathway is intact in human pulmonary artery endothelial cells, dominant survival signaling protects these cells from TNF induced apoptosis [9]. However, the mechanism of this resistance is incompletely understood. |
| |
| Endothelial cells possess two TNFα receptors, TNFR1 (55-60 kD) and TNFR2 (75-80 kD); both pro-apoptotic and anti-apoptotic signals are regulated principally by TNFR1 [10]. We recently illustrated the role of the actin cytoskeleton in NFκB activation and proposed that TNFα- induced cytoskeletal rearrangement preferentially activates NFκB- dependent survival signaling [9,11]. However, the signaling pathways linking cytoskeletal rearrangement and the induction of NFκB- dependent survival are not yet clear. An important regulatory step in the regulation of anti-apoptotic signaling is the assembly of the TNFR1-TRADD-TRAF2-RIP complex, which controls NFκB activation [12]. Xia et al. have reported a direct interaction of SphK with TRAF2 [8,13], suggesting a role for SphK in NFκB activation. It was proposed that TNFα-induced activation of NFκB and JNK most likely bifurcates at the site of TRAF2 binding [14]. While SphK mediated TRAF2- promoted NFκB activation, JNK activation was SphK-independent. Neither wild-type SphK nor the dominant-negative SphK influenced JNK activity induced either by TNFα and/or TRAF2 [15,16]. The association of SphK1 with the TRAF2 complex and the subsequent activation of NFκB dependent signaling strongly suggests that S1P generation is a necessary step in survival signaling, and not only does it involve its cognate receptors, but the TNFR receptor and growth factor receptors such as PDGF and EGF as well [17,18]. |
| |
| Earlier we have shown that human pulmonary artery endothelial cells are normally resistant to several apoptotic effects of stimuli associated with lung disease and one of the survival pathways induced is NFkB dependent [9]. The regulation of lipid raft assembly in HPAE cells during TNF induced survival signaling is not well characterized. It has been reported that in human fibrosarcoma HT1080 cells, TNFR1 translocates to lipid rafts within 2 min after TNFα binding [19]. Subsequently, the TRADD–TRAF2–RIP complex forms and initiates the signaling pathway leading to NFκB activation. Disruption of the lipid rafts by cholesterol depletion inhibits activation of NFκB, and apoptosis is induced. This suggests that lipid rafts serve as platforms for cellular signaling through TNFR1 [20]. It has also been reported that LPS activates macrophages by binding to the receptor TLR4, leading to the production of cytokines [21], and that lipid raft integrity is essential for LPS-cellular activation, since raft-disrupting drugs block the signaling pathway [22]. |
| |
| To evaluate the relationship between SphK-TNFR signaling in pulmonary artery endothelial cell plasma membrane lipid raft microdomains, we first localized SphK1 to the lipid raft enriched cellular fractions. After TNFα treatment in HPAE cells, cytoplasmic SphK1 was localized to the membrane fraction. Furthermore, we identified an inducible interaction between Lyn kinase and SphK1, localized to the lipid rafts. Here, we demonstrate that during TNFR receptor signaling, SphK1 association with the TNFR complex and its translocation to the lipid rafts plays an important role in SphK activation and S1P synthesis. We further correlate S1P synthesis with the induction of survival signaling that protect the HPAECs from TNF induced cell death. |
| |
| Materials and Methods |
| |
| Western blot analysis |
| |
| Equal amounts of nuclear or cytoplasmic proteins were separated on 10 % precast Tris-HCl gels (Bio-Rad) under denaturing condition. Proteins were electro-blotted to nitrocellulose membranes and incubated with various antibodies depending on the assay. The control antibodies used for cytoplasmic proteins were anti- actin or GAPDH. |
| |
| Immunocytochemistry |
| |
| HPAEC cells were grown on 1% gelatin coated cover-slips. Cells were washed twice in PBS, fixed with 4% formaldehyde rinsed again with PBS and incubated in permeabilization solution (0.1% Triton X-100, 0.1% Sodium citrate) for 5 minutes on ice. After blocking in 3%BSA in PBS at 4°C for 1 hr, cells were incubated sequentially in an anti-SphK1, TRAF2, TRADD primary antibody overnight and secondary antibody conjugated to fluorescein (Vector Laboratories, Burlingame, CA) for 1 hr in the dark. They were then rinsed three times in PBS, mounted with a medium containing DAPI (for nuclear staining) and visualized with a fluorescent microscope. |
| |
| Cell culture and siRNA transfection |
| |
| HPAEC cells were cultured in complete medium (DMEM medium supplemented with 10% fetal bovine serum (FBS), 2 mM -glutamine, and 100 U/ml penicillin and streptomycin). A 21-mer Lyn kinase siRNA oligonucleotide (Ambion); 5’-CUAGAGUAAUUGAAGAUAAtt-3’ (sense), and 5’-UUAUCUUCAAUUACUCUAGca-3’ (antisense), was used to target Lyn kinase mRNA. The siRNA was diluted in Opti- MEM (Invitrogen) media and transfected into cells grown to 70-90% confluence, using Lipofectamine 2000 reagent (Invitrogen). After transfection, cells were incubated at 37°C and 5% CO2 in DMEM medium supplemented with 10% FBS and 1% glutamine in the absence of antibiotics. Cells were harvested after 48 hours of siRNA transfection for mRNA quantitation. For control, cells were transfected with the scrambled siRNA sequence. |
| |
| Transfection and luciferase assay |
| |
| Endothelial cells (EC) were seeded (2×105cells/well) in six-well plates and were transfected using the Lipofectamine transfection method (BRL Lab) with a total of 6μg of DNA/plate and transfected in triplicate. The EC were co-transfected with a luciferase reporter gene construct (1 μg/well), regulated by five copies of the consensus sequence of NF-κB DNA binding site [(NFkB) 5-Luc] (Stratagene). Cells were also co-transfected with a cytomegalovirus early promoter driven beta-galactosidase gene (CMV bgal) and the data was normalized to the activity of beta-galactosidase activity. Cell lysates were prepared for luciferase and -galactosidase (βgal) activity assays as per manufacturer's instructions 24-48 h after transfection (Promega, Madison, WI). |
| |
| Isolation of lipid rafts by sucrose density centrifugation |
| |
| Control and TNFα treated HPAE cells grown to confluency were harvested in 500mM Na2CO3 and homogenized and sonicated. Samples were then mixed in equal volumes with a 90% sucrose/MES (2-(N-morpholino) ethanesulfonic acid) -buffered saline (MBS), yielding a 45% sucrose/MBS solution. 4mL of the 45% sucrose solution containing the samples was then transferred to an ultracentrifuge tube, with 4mL of 35% sucrose in MBS/Na2CO3 layered above, and finally 4mL of 5% sucrose in MBS/Na2CO3 on top. Samples were then centrifuged at 39,000 RPM for 20hrs at 4°C, with a maximum force of approximately 260,000g. Following centrifugation, 1mL samples were removed from the top of the tube down (with fraction #1 at the top of the tube and fraction #12 at the bottom), and stored for analysis. |
| |
| Apoptosis assays |
| |
| Endothelial cell death staining: HPAE cells were stained with Hoechst 33342 dye and propidium iodide (Roche molecular system, N.J.). Detached cells were recovered by centrifugation, resuspended in binding buffer, and added back to the tissue culture plate before being stained. Apoptotic cells were detected by fluorescence microscopy with an Eclipse TE300 inverted microscope (Nikon; Melville, NY). Hoechst 33342 binds preferentially to adenine-thymine (A-T) regions of DNA. This stain binds into the minor groove of DNA and exhibits distinct fluorescence emission spectra that are dependent on dye:base pair ratios. Apoptotic cells stained blue at the plasma membrane, while necrotic or late apoptotic cells took up propidium iodide and stained red. A quantitative estimation was made by counting apoptotic cells relative to the total number of cells seen within the counted fields (with the use of bright-field microscopy). |
| |
| Caspase-8 apoptosis assay |
| |
| Apoptosis was induced in control, empty vector adenovirus, SphK1KD, and SphK1-overexpressing HPAE cells by treatment with TNFα (100ng/mL) for 24 hrs. Cells were harvested and pelleted, then incubated in Cell Lysis Buffer (BioVision) for 10 mins on ice. Cells were then centrifuged again for 1 min at 10,000xg, and the supernatant transferred to a new tube and put on ice. Supernatant samples were then mixed with a Reaction buffer (containing 10mM DTT) and a 200μM IETD-pNA substrate (BioVision), and incubated at 37°C for 2 hrs. Samples were analyzed at 405nm for optical density using a spectrophotometer, and percentages of apoptotic cells were calculated. |
| |
| Statistical analysis |
| |
| Each experiment was conducted at least five times. Data is typically expressed as mean ± S.D. Data between two groups were analyzed by Student’s t test and among multiple groups by ANOVA followed by the Student-Newman-Keuls (SNK) test. A p value of less than 0.05 was considered significant. |
| |
| Results |
| |
| Over-expression of SphK1 and SphK2 in HPAE cells and its effect on intracellular S1P generation |
| |
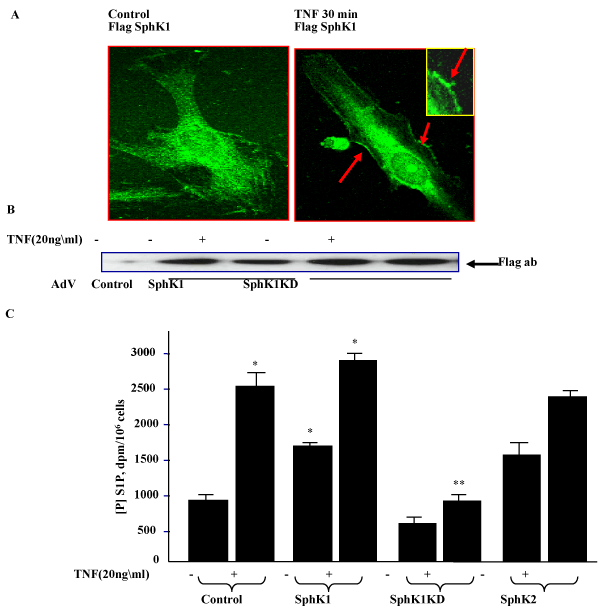
| We investigated the effects of over-expression of wild-type SphK1 & SphK2, and a catalytically inactive mutant (SphK1KD) on intracellular S1P generation. Vector control, SphK1, SphK2, or SphK1KD overexpressing cells were labeled with [32P] - orthophosphate for 3hr, then exposed to TNFα (20ng/ml) for 30 min. As shown in Figure 1, TNFα treatment induced S1P generation in HPAE cells and overexpression of wild-type SphK1 alone increased intracellular generation of [32P] S1P and was further stimulated by TNFα treatment. However, overexpression of the SphK1KD significantly attenuated the response, suggesting that it acts like a dominant negative mutant blocking both intracellular kinases. These results demonstrate the utility of the adenoviral expression vectors in studies related to the role of SphKs in S1P formation and signaling. TNFα treatment further increased S1P generation in SphK1 overexpressing cells, suggesting that activation of sphingosine kinase was crucial for S1P generation. |
| |
|
|
Figure 1: Effect of over-expression of SphK 1and 2 wild-type and mutants in HPAECs and its effect on intracellular S1P generation in HPAE cells: A. HPAECs cells were infected with either empty vector, SphK1 wild-type or SphK1KD mutant expressing adenoviruses. 48hr post infection cells were fixed and immunostained for SphK expression using either a Flag or a SphK1- specific antibody. B. SphK1 and SphK1KD expression following viral infection and TNFα treatment (20ng/ml) was detected by Flag antibody staining. C. 48 hr post infection, cells were labeled with 32P Orthophosphate and exposed to TNFα for 1hr and cellular S1P generation was assayed from cytosolic fractions using TLC, as described earlier [26]. |
|
| |
| Distribution of SphK-TNFR complexes in endothelial cell lipid rafts |
| |
| |
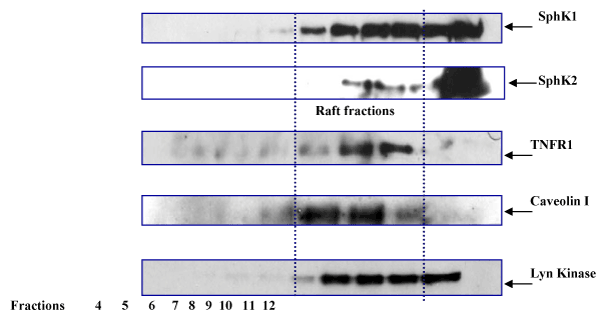
| Recent studies have shown that members of the TNFR superfamily, including CD40 and TNFR1, become associated with lipid rafts following ligand binding [20], although TNFR1 can be localized to non-raft fractions as well [23]. However, in HPAECs it is not clear if SphK1 activation is dependent on translocation to lipid rafts. Using adenoviral expression vectors, we overexpressed Flag-tagged-SphK1 and c-myc-tagged-SphK2 in HPAE cells. 48hr post- transfection, the cells were treated with TNFα (20ng/ml) for 30 min. Membrane and cytosolic fractions were prepared by sucrose density gradient centrifugation and protein in all fractions was characterized by western blotting. Fractions expressing caveolin-1 were determined to be plasma membrane lipid raft fractions, as caveolin is a major structural component of lipid raft microdomains. Following TNFα treatment, we observed significant levels of SphK1 associated with lipid raft fractions, along with co-localization of TNFR1 expression in these fractions as well (Figure 2). In HPAE cells, immunofluorescence staining localized SphK2 expression to the nuclear region, however after TNFα treatment we identified SphK2 expression in lipid raft fractions as well, suggesting a novel role for this kinase in membrane signaling. As expected, Lyn kinase, one of the markers for lipid microdomain proteins, was present in these fractions. These results suggest that both SphK1 and SphK2 translocate to lipid raft in response to TNFα treatment. |
| |
|
|
Figure 2:Association of SphK1 and SphK2 with lipid raft domains: HPAE cells infected with SphK1-Flag and SphK2-myc expressing adenoviruses were treated with TNFα for 1hr, membrane and cytoplasmic fractions were prepared by sucrose density gradient (as described in Materials and Methods). Fractions were separated on 4-12% gradient gels and immunoblotted for Flag, c-myc, TNFR1 and Caveolin1 antibodies. SphK1, SphK2 and TNFR1 proteins are localized into raft fractions. |
|
| |
| Translocation of SphK1 in lipid raft fractions, and functional interaction with Lyn kinases |
| |
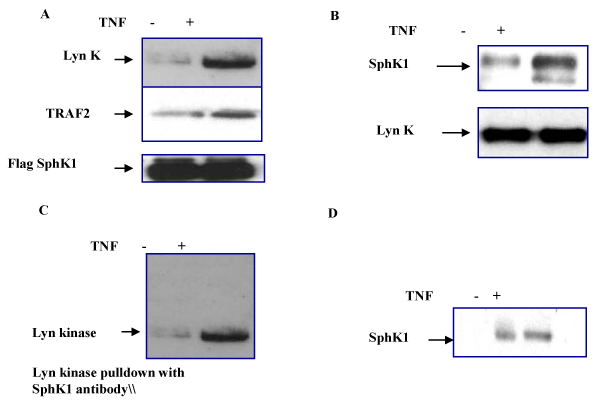
| As we noted that TNFα treatment induces SphK1 translocation to the lipid raft fractions, we next attempted to identify the cellular SphK1 binding partners in endothelial membrane microdomains. For this purpose, we first overexpressed Flag-SphK1 in HPAE cells and using Flag antibody pull down experiments we identified that Lyn kinase and TRAF2 protein binds to Flag-SphK1 (Figure 3A). Flag-beads alone did not show any non-specific binding to Lyn kinase. We next identified a direct interaction of SphK with Lyn kinase, using a Lyn kinase specific antibody which does not recognize Fyn kinase family members or other tyrosine kinases. In the reverse IP experiments we used a Lyn kinase specific antibody for immunoprecipitation and immunoblotted Flag antibody to detect SphK1 (Figure 3B). These results suggest that TNFα- induced SphK activation specifically induced the Lyn kinase binding. Further using SphK1 specific antibody we immunoprecipitated lyn kinase (Figure 3C) which suggest a direct interaction of SphK1 with lyn kinases in the lipid raft fractions. After TNF treatment, Lyn kinase autophosphorylation was enhanced but significant SphK1 phosphorylation was not observed (data not shown). These results suggest that SphK1 may not be a substrate for Lyn kinase but instead however, may serve as an important anchor for SphK activation in lipid raft fractions. |
| |
|
|
Figure 3:Identification of Lyn kinase SphK interaction in endothelial cells lipid raft fractions: HPAECs expressing Flag-SphK1 were treated with TNFα for 30 min and raft fractions were prepared by sucrose density gradient. A. Caveolin-1 enriched fractions were immunoprecipitated using Flag antibody and western blotted for Lyn kinase and TRAF2 binding using Lyn kinase and TRAF2 specific antibodies. B. Using Lyn kinase antibody, Caveolin-1 enriched fractions were immunoprecipitated and western blotted by sphK1 specific antibody as described in Methods C. In the reverse immunoprecipitation using SphK1 specific antibody Lyn kinase was immunoprecipitated. D. Lyn kinase expressing fractions were collected and immunoprecipitated for Flag-SphK1 and western blotted using phospho-tyrosine specific antibody. TNF treatment induced SphK phosphorylation. |
|
| |
| Role of SphK-Lyn kinase interaction in S1P synthesis |
| |
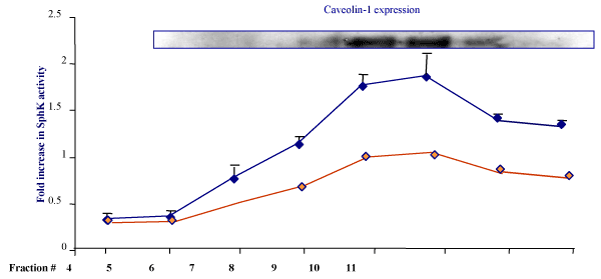
| Based on previously discussed observations, we asked if association of SphK1 with Lyn kinase enhances the TNF- dependent S1P synthesis in the lipid raft fractions of endothelial cells. In mast cells, SphK1 activity was increased in response to IgE treatment in lipid rafts [24]. Increased Lyn kinase activity and phosphorylation has been also documented previously in mast cells [25]. However, if endothelial SphK activity enhances in response to TNFα treatment in lipid rafts is not known. Therefore, we first assessed the overall cellular localization of SphK activity in all fractions. HPAE cell lysates after TNFα treatment (30 min. 20ng/ml) were fractionated using a sucrose density gradient centrifuge. As shown in Figure 2, caveolin-1 expressing fractions were monitored for S1P synthesis. Total SphK1 activity was measured as described earlier (Wadgaonkar et al. [26] and fold increase in S1P synthesis after TNFα treatment were monitored in fractions 4-11 (Figure 4). Next to understand if Lyn kinase interaction is required for SphK1 activiation in the lipid raft microdomains we attenuated expression of Lyn kinase using siRNA specific for human Lyn kinase as described in the Methods. At 100μM concentration of siRNA oligo we found 80-90 % inhibition of Lyn expression. This concentration was used for HPAE transfection and SphK1 activity was measured from siRNA treated fractions. There was significant decrease in the activity of SphK1 was noticed in the Caveloin enriched fractions. This suggests that SphK1 translocation as well as its interaction with Lyn was involved in SphK1 activation. |
| |
|
|
Figure 4:TNFα induced SphK1 redistribution and S1P synthesis in lipid raft fractions: HPAE cells confluent monolayer treated with TNFα (20ng/ml) for 30 min and cell lysates were fractionated using sucrose density gradient. A. Fractions were immunoblotted for Caveolin-1 antibody. B. SphK1 kinase activity was determined using 3H-sphingosine as a substrate in lipid raft fractions and total S1P synthesis was measured by TLC as described earlier [26] and fold increases in activity was determined (Blue bars n=3 experiments averaged for each point). As described in Methods Lyn Kinase expression was attenuated using Lyn specific siRNA (100μM) and SphK1 kinase activity was determined (red bars n=3 experiments) |
|
| |
| Disruption of microdomains induces redistribution of SphK |
| |
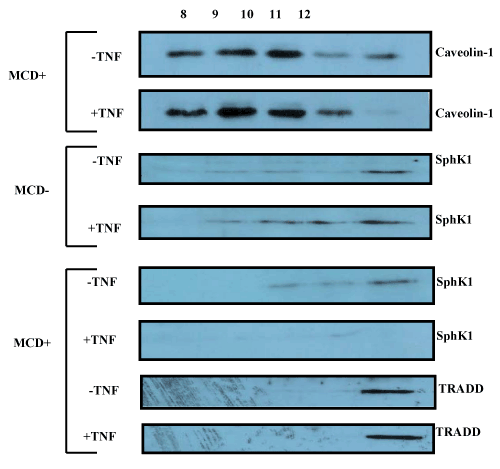
| Using methyl-β-cyclodextrin (MCD) that depletes cholesterol, we disrupted the lipid rafts in order to determine the SphK1 distribution. We found that with MCD treatment, SphK1 expression was significantly less in the caveolin fractions after TNFα treatment. We also found that TRADD does not redistribute to the caveolin fractions (Figure 5). |
| |
|
|
Figure 5:Effect of SM microdomain disruption on SphK1 translocation: As described in Methods, using methyl-β-cyclodextrin (MCD) treatment lipid rafts were disrupted and Caveolin 1, SphK1 and TRADD expression was determined in lipid raft fractions. After MCD treatment, followed by TNFα exposure, SphK1 expression was significantly less in the caveolin fractions. MCD or TNFα treatment did not redistribute the TRAF2 binding TRADD protein to the caveolin fractions. |
|
| |
| Effect of inhibition of S1P synthesis on HPAE cell survival |
| |
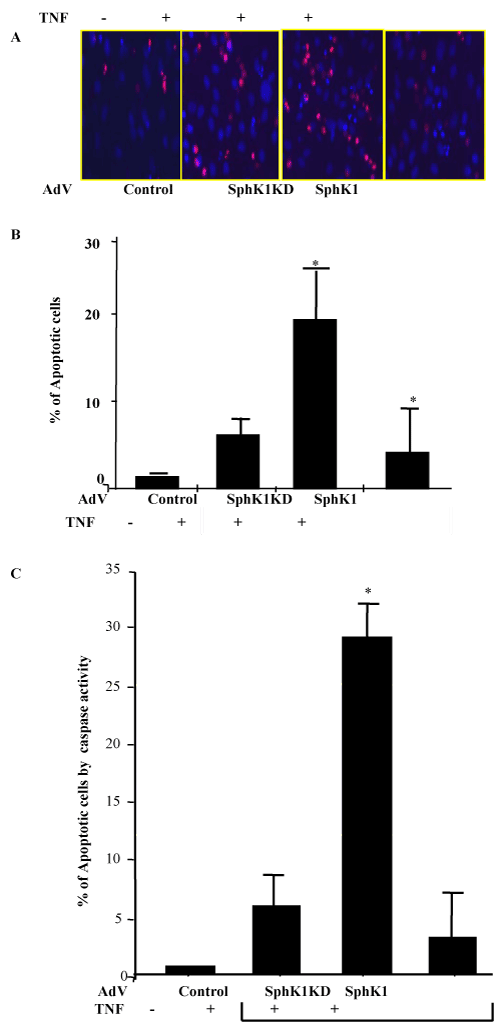
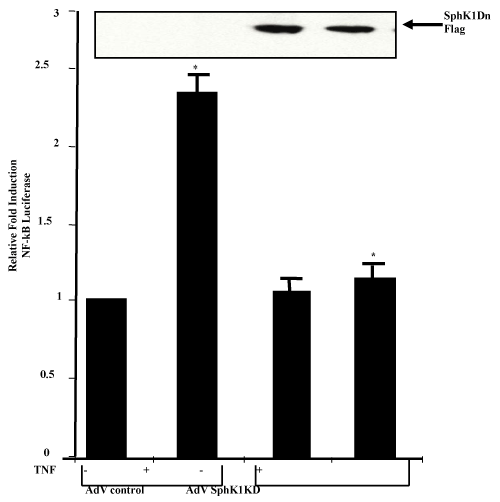
| To understand if SphK1 translocation to the lipid raft microdomains and S1P synthesis is responsible for endothelial cell protections, we studied endothelial cell apoptosis. First, we overexpressed SphK1 and SphK1 dominant negative mutant in HPAE cells, and treated them with TNFα for 24 hours. Apoptotic cell death was measured using several assays including Hoechst 33342 and propidium iodide staining, caspase 8 activity assay combining with flow cytometric and fluorescence imaging. We found that TNFα treatment alone did not significantly induce apoptosis. However, overexpression of the SphK1 kinase dead mutant (SphK1KD) after TNFα treatment showed an increase in apoptosis, while wild type SphK1 overexpression did not induce apoptosis in response to TNFα (Figure 6A-C). In HPAE cells, treatment with high doses of TNFα (100ng/ml) for 24 hr does not induce apoptosis. This led us to ask, what mechanisms are involved for protecting endothelial cells from apoptosis. We then studied if SphK1KD have any effect on NFκB activation. TNF induced NFκB dependant transactivation was significantly lower in SphK1KD expressing cells, suggesting that the SphK dominant negative mutant may induce apoptosis through inhibition of NFκB -dependant survival signaling (Figure 7). |
| |
|
|
Figure 6:Effect of TNFα treatment on endothelial cells apoptosis: HPAEC confluent monolayer infected with adnoviruses expressing empty vector, SphK1, SphKKD, were treated with TNFα for 24hrs and apoptotic cell death was measured by two different techniques as described earlier [11]. A. After 24hrs of TNF treatment HPAE cells were stained with Hoechst 33342 and propidium iodide for 30 min. cells were imaged using Fluorescence imaging. Total number of apoptotic, late apoptotic cells were counted from 3-6 fields and averaged per plate (n=3 plates/each condition). B. % percentage increase in apoptotic cells after TNF treatment were averaged and fold increase in apoptosis for each condition was determined. * represents total increase or decrease in apoptosis as compare to control cells treated with TNF. C. Caspase 8 activity assay: Apoptosis was induced in control, empty vector adenovirus, SphK1KD, and SphK1-overexpressing HPAE cells by treatment with TNFα (100ng/mL) for 24 hrs. Cells were harvested and pelleted, then incubated in Cell Lysis Buffer (BioVision) for 10 mins on ice. Caspase activity assay performed as described in Methods. Data are presented as representative of three independent experiments. TNFα treatment alone did not significantly induce apoptosis. However, overexpression of the SphK1 kinase dead mutant after TNFα treatment showed an increase in apoptosis, while wild type SphK1 overexpression did not induce apoptosis in response to TNFα treatment. |
|
| |
|
|
Figure 7:Effect of SphKD overexpression on TNF induced NFκB transactivation: A. HPAE cells at 70-80% confluency were transfected with NFκB Luciferase reporter plasmid and sphingosine kinase dominant negative mutant expression vector. Forty eight hours post-transfection, cells were treated with TNFα (20ng/ml) for eight hours. Cells were harvested after 8 hr and analyzed for luciferase activity and SphK1KD expression was monitored by western blotting. |
|
| |
| Discussion |
| |
| Effect of SPHK1 redistribution on S1P generation in caveolin enriched fraction |
| |
| To evaluate the relationship between SphK-TNFR signaling with membrane lipid rafts, we first have localized SphK1 and SphK2 kinases in raft fractions. After TNFα treatment in HPAE cells, cytoplasmic SphK1 was localized to membrane fraction. Furthermore, we identified inducible interaction between SphK1 and Lyn kinase and localized SphK1 to the lipid rafts. Next, we observed translocation of nuclear SphK2 to membrane fraction. These results suggest that, after TNFα treatment, SphK1 and 2 both translocate to the membrane. To understand the role of SphK1 translocation to the lipid raft fractions, we focused on an endothelial lipid raft associated protein which may interact with SphK1. Earlier studies have identified SphK interaction with Lyn kinases in mast cells [24]. This interaction was shown to be involved in IgE secretion and was enhanced after TNF treatment. |
| |
| SphK-Lyn kinase interaction in microdomain may be required for S1P generation |
| |
| In HPAE cells, we identified SphK1 translocation to the microdomains in response to TNFα treatment, which may be important for SphK activation and binding to Lyn Kinase. This may be independent of tyrosine phosphorylation by Lyn kinase, since SphK phosphorylation was not significantly increased. Also, after TNFR signalosome complex translocation into lipid raft, Lyn-SphK activation may be enhanced because of proximity to lipid rich areas. Functional interaction of SphK1 with the tyrosine kinase- Lyn links SphK activation and S1P synthesis to a downstream TNFR signaling (Figure 4). |
| |
| Effect of S1P generation on endothelial cell survival |
| |
| To test the hypothesis that basal level S1P synthesis is required to promote survival of HPAEC and thus contributes to their resistance to proinflammatory stressors, using SphK1KD dominant negative kinase, we suppressed the S1P synthesis and then evaluated changes in basal cell death and sensitivity to apoptotic stimuli caused by this suppression. Similar to what has been reported in the literature for HUVEC [27] and HDMEC [28], our data show that HPAEC exhibit an inherent resistance to multiple potential apoptotic stimuli. Studies in animals and humans indicate that pulmonary endothelium apoptosis likely contributes to the dysfunction of the vascular endothelium associated with sepsis and sepsis-induced organ failure. Biopsy and autopsy samples from humans with ALI/ARDS secondary to sepsis demonstrate increased endothelial cell apoptosis compared with control lung tissue. Recent studies have shown that endotoxemia and gram-negative sepsis result in apoptosis in the gut, lymphoid tissue, and lung [29]. In the mice model of sepsis specifically, reveals apoptosis of pulmonary vascular EC [30]. Systemic administration of apoptosis inhibitors improves survival in animal models of endotoxin-induced injury, suggesting that apoptotic cell death contributes causally to disease-related mortality [31]. Our Experiments strongly suggest that TNFα induced S1P generation protects endothelium from apoptosis, which support the earlier observations [32]. |
| |
| The mechanism of survival signaling is via SphK1-dependent NFκB activation |
| |
| It is now established that the majority of the cytotoxic effects of TNFα are mediated by TNF-receptor-1 (TNFR1) through the interaction of its death domain protein, TRADD [33,34]. The characterization of TRADD deficient mice demonstrated that TRADD was essential for TNFR1 induced apoptosis. TRADD deficiency inhibited TNFR1- induced cell death in vivo and in vitro, which demonstrated that TRADD was essential for TNFR1-induced apoptosis [34]. After TNFα treatment endothelial TRADD was not localized with TRAF2 or SphK1-Lyn complexes in the raft fractions suggesting that SphK1 dependent survival signaling complex does not required TRADD interaction. Also, suggesting the mechanism of resistance present in the pulmonary endothelial cells against TNFα induced apoptosis. |
| |
| In conclusion, we propose that in pulmonary endothelium, SphK1 association with the TNFR complex and its translocation to the lipid rafts plays an important role in S1P synthesis. Endothelial cell protection against TNF alpha induced death signaling is regulated by early activation of SphK1 and recruitment of the survival signaling complex to the lipid rafts and which results into the induction of survival signaling that protect the HPAECs from cell death. |
| |
| Funding Resources |
| |
| VA Merit Award (RW), ECRIP Award (RW,SG). |
| |
| |
| References |
| |
- Kolesnick R (1994) Signal transduction through the sphingomyelin pathway. Mol Chem Neuropathol 21: 287-297.
- Mathias S, Kolesnick R (1993) Ceramide: a novel second messenger. Adv Lipid Res 25: 65-90.
- Hannun YA (1996) Functions of ceramide in coordinating cellular responses to stress. Science 274: 1855-1859.
- Pronk GJ, Ramer K, Amiri P, Williams LT (1996) Requirement of an ICE-like protease for induction of apoptosis and ceramide generation by REAPER.Science 271: 808-810.
- Goggel R, Winoto-Morbach S, Vielhaber G, Imai Y, Lindner K, et al. (2004) PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med 10: 155-160.
- Maceyka M, Payne SG, Milstien S, Spiegel S (2002) Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta 1585: 193-201.
- Hla T (2003) Signaling and biological actions of sphingosine 1-phosphate. Pharmacol Res 47: 401-407.
- Xia P, Gamble JR, Rye KA, Wang L, Hii CS, et al. (1998). Tumor necrosis factor-a induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci U S A 95: 14196-14201.
- Wadgaonkar R, Linz-McGillem L, Zaiman AL, Garcia JG (2005) Endothelial cell myosin light chain kinase (MLCK) regulates TNFalpha-induced NFkappaB activity. J Cell Biochem 94: 351-364.
- Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA Jr, et al. (1991) The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A 88: 9292-9296.
- Petrache I, Birukov K, Zaiman AL, Crow MT, Deng H, et al. (1993) Caspase-dependent cleavage of myosin light chain kinase (MLCK) is involved in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. FASEB J 17: 407-416.
- Legler DF, Micheau O, Doucey MA, Tschopp J, Bron C (2003) Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity 18: 655-664.
- Xia P, Wang L, Gamble JR, Vadas MA (1999) Activation of sphingosine kinase by tumor necrosis factor-alpha inhibits apoptosis in human endothelial cells. J Biol Chem 274: 34499-34505.
- Wadgaonkar R, Pierce JW, Somnay K, Damico RL, Crow MT, et al. (2004) Regulation of c-Jun N-terminal kinase and p38 kinase pathways in endothelial cells. Am J Respir Cell Mol Biol 31: 423-431.
- Di A, Kawamura T, Gao XP, Tang H, Berdyshev E, et al. (2010) A Novel Function of Sphingosine Kinase 1 Suppression of JNK Activity in Preventing Inflammation and Injury. J Biol Chem 285: 15848-15857.
- Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, et al. (2003) Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J 22: 5491-5500.
- Kluk MJ, Colmont C, Wu MT, Hla T (2003) Platelet-derived growth factor (PDGF)-induced chemotaxis does not require the G protein-coupled receptor S1P1 in murine embryonic fibroblasts and vascular smooth muscle cells. FEBS Lett 533: 25-28.
- Le Stunff H, Mikami A, Giussani P, Hobson JP, Jolly PS, et al. (2004) Role of sphingosine-1-phosphate in epidermal growth factor-induced chemotaxis. J Biol Chem 279: 34290-34297.
- Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387: 569-572.
- Legler DF, Micheau O, Doucey MA, Tschopp J, Bron C (2003) Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity 18: 655-664.
- Triantafilou M, Miyake K, Golenbock T, Triantafilou K (2002) Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci 115: 2603-2611.
- Van der Luit AH, Budde M, Zerp S, Caan W, Klarenbeek JB, et al. (2007) Resistance to alkyl-lysophospholipid-induced apoptosis due to downregulated sphingomyelin synthase 1 expression with consequent sphingomyelin- and cholesterol-deficiency in lipid rafts. Biochem J401: 541-549.
- Hunter I, Nixon GF (2006) Spatial compartmentalization of tumor necrosis factor (TNF) receptor 1-dependent signaling pathways in human airway smooth muscle cells. Lipid rafts are essential for TNF-alpha-mediated activation of RhoA but dispensable for the activation of the NF-kappaB and MAPK pathways. J Biol Chem 281: 34705-34715.
- Urtz N, Olivera A, Bofill-Cardona E, Csonga R, Billich A, et al. (2004) Early activation of sphingosine kinase in mast cells and recruitment to FcepsilonRI are mediated by its interaction with Lyn kinase. Mol Cell Biol 24: 8765-8777.
- Olivera A, Urtz N, Mizugishi K, Yamashita Y, Gilfillan AM, et al. (2006) IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J Biol Chem 281: 2515-2525.
- Wadgaonkar R, Patel V, Grinkina N, Romano C, Liu J, et al. (2009) Differential Regulation of Sphingosine Kinases 1 and 2 in Lung injury. Am J Physiol Lung Cell Mol Physiol 296: L603-L613.
- Bannerman DD, Tupper JC, Ricketts WA, Bennett CF, Winn RK, et al. (2001) A constitutive cytoprotective pathway protects endothelial cells from lipopolysaccharide-induced apoptosis. J Biol Chem 276: 14924-14932.
- Zen K, Karsan A, Stempien-Otero A, Yee E, Tupper J, et al. (1999) NF-kappa B activation is required for human endothelial survival during exposure to tumor necrosis factor-alpha but not to interleukin-1beta or lipopolysaccharide. J Biol Chem 274: 28808-28815.
- Choi KB, Wong F, Harlan JM, Chaudhary PM, Hood L, et al. (1998) Lipopolysaccharide mediates endothelial apoptosis by a FADD-dependent pathway. J Biol Chem 273: 20185-20188.
- Hu X, Yee E, Harlan JM, Wong F, Karsan A (1998) Lipopolysaccharide induces the antiapoptotic molecules, A1 and A20, in microvascular endothelial cells. Blood 92: 2759-2765.
- Miyaji M, Jin ZX, Yamaoka S, Amakawa R, Fukuhara S, et al. (2005) Role of membrane sphingomyelin and ceramide in platform formation for Fas-mediated apoptosis. J Exp Med 202: 249-259.
- Castillo SS and Teegarden D (2003) Sphingosine-1-phosphate inhibition of apoptosis requires mitogen-activated protein kinase phosphatase-1 in mouse fibroblast C3H10T
 cells. J Nutr 133:3343-3349. cells. J Nutr 133:3343-3349.
- Hsu H, Xiong J, Goeddel DV (1995) The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 81: 495-504.
- Ermolaeva MA, Michallet MC, Papadopoulou N, Utermöhlen O, Kranidioti K, et al. (2008) Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nat Immunol 9: 1037-1046.
|
| |
| |