| Research Article |
Open Access |
|
| Amr M Badawey, Nadia M Mostafa, Nesrine T Lamie* and Abd El-Aleem AEB |
| Analytical Chemistry Department, Faculty of Pharmacy, Cairo University, Kasr El-Aini St., ET11562 Cairo, Egypt |
| *Corresponding author: |
Nesrine T Lamie
Analytical Chemistry Department
Faculty of Pharmacy, Cairo University
Kasr El-Aini St., ET11562 Cairo, Egypt
Tel: +201222711259
E-mail: nesrinelamie@hotmail.com |
|
| |
| Received August 27, 2012; Published September 22, 2012 |
| |
| Citation: Badawey AM, Mostafa NM, Lamie NT, Abd El-Aleem AEB (2012) Stability-Indicating Methods for the Determination of Rosuvastatin in the Presence of its Oxidative Degradation Products. 1:322. doi:10.4172/scientificreports.322 |
| |
| Copyright: © 2012 Badawey AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Four different accurate, sensitive and reproducible stability-indicating methods for the determination of rosuvastatin calcium in the presence of its oxidative degradation products are presented. The first method is Second-derivative (2D) method at 243.6 nm in a concentration range of 5-30 μg mL−1 with mean percentage recovery of 99.94±1.171. The second method is based on ratio-spectra 1st derivative (1DD) spectrophotometry of the drug at 240 nm, over a concentration range of 5-35 μg mL−1 with mean percentage recovery of 99.77±0.974. The third method utilizes quantitative densitometric evaluation of thin-layer chromatography of rosuvastatin calcium in the presence of its oxidative degradation products, using ethyl acetate: methanol: ammonia (7:3:0.01, v/v/v) as a mobile phase. Chromatograms are scanned at 245 nm. This method analyses rosuvastatin calcium in a concentration range of 0.6-3.4 μgspot-1with mean percentage recovery of 99.78±1.419. The fourth method is an HPLC method for the simultaneous determination of rosuvastatin calcium in the presence of its oxidative degradation products. The mobile phase consists of water: acetonitrile: methanol (40: 40: 20 by volume). The standard curve of rosuvastatin calcium shows a good linearity over a concentration range of 10- 60 μg mL-1 with mean percentage recovery of 100.22±0.859. These methods were successfully applied to the determination of rosuvastatin calcium in bulk powder, laboratory-prepared mixtures containing different percentages of the degradation products and pharmaceutical dosage forms. The validity of results was assessed by applying standard addition technique. The results obtained were found to agree statistically with those obtained by a reported method, showing no significant difference with respect to accuracy and precision. |
| |
| Keywords |
| |
| Rosuvastatin calcium; Stability-indicating; Ratio-spectra; Derivative; Densitometry; HPLC technique |
| |
| Introduction |
| |
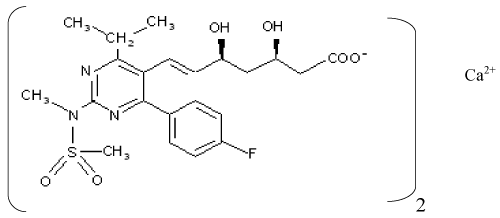
| Rosuvastatin; bis ((E)-7-(4-(4-flurophenyl)-6-1sopropyl-2-(methyl (methylsulfonyl) amino) pyrmidin-5yl)(3R,5S)-3,5-dihydroxyhept-6- enoic acid) calcium salt, (Figure 1) is a highly effective 3-hydroxyl- 3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor. It is widely used for the treatment of hyperlipidemia.In clinical trials, rosuvastatin achieved marked reductions in serum levels of LDL cholesterol, accompanied by modest increases in HDL cholesterol and reductions in triglycerides [1-3]. |
| |
|
|
Figure 1: Chemical structure of rosuvastatin calcium. |
|
| |
| It may also be used in patients with homozygous familial hypercholesterolaemia. Rosuvastatin is given orally as the calcium salt, although the doses are expressed in terms of the base. |
| |
| Despite of the wide application of rosuvastatin in the treatment of hyperlipidemia, a literature survey reveals that only few methods have been reported for the determination of RC in pharmaceutical formulation and biological samples including HPLC [4-8], spectrophotometry [9] capillary electrophoresis [10] and chemometry [11]. |
| |
| An ideal stability indicating method is one that quantifies the standard drug alone and also resolves its degradation products [12]. None of these methods is concerned with the Identification and elucidation of the structure of the resulting oxidative degradation products, thus the objective of the present study was to develop simple and accurate stability -indicating methods for selective determination of rosuvastatin calcium in the presence of its oxidative degradation products with the application to pharmaceutical dosage forms that could be applied for drug quality control. |
| |
| Experimental |
| |
| Apparatus |
| |
| Spectrophotometer: Shimadzu UV-1601 PC, dual-beam UVvisible spectrophotometer (KyotoJapan), with matched 1cm quartz cells, connected to an IBM-compatible PC and an HP-600 inkjet printer. Bundled, UV-PC personal spectroscopy software version 3.7, was used to process the absorption and the derivative spectra. The spectral band width was 2nm with wavelengthscanning speed of 2800 nm min−1. |
| |
| IR Spectrophotometer: Shimadzu 435 (Kyoto, Japan), sampling was undertaken as potassium bromide discs. |
| |
| Ultra Performance Liquid Chromatography - Mass Spectrophotometer (UPLC MS-MS), Acquity TQ-Waters (USA); used for mass spectrophotometric analysis. |
| |
| Precoated TLC-plates, silica gel 60 F254 (20cm×20 cm, 0.25 mm), E. Merck (DarmstadtGermany). Camag TLC scanner 3 S/N 130319 with winCATS software. Camag Linomat 5 autosampler (Switzerland). Camag microsyringe (100 μL). |
| |
| A liquid chromatograph consisted of an isocratic pump (Shimadzu LC-10 AD) an ultraviolet visible wavelength detector (SPD-10A, Shimadzu), a Rheodyne injector (Model 7725 I, Rohnert Park, CA, USA) equipped with 20 μL injector loop. Stationary phase; a 250 mm × 4.6 mm C18, i.d. 5 μm analytical column, Waters (USA). The mobile phase water: acetonitrile: methanol (40: 40: 20 by volume) was filtered through a 0.45 μm Millipore membrane filter and was degassed for 15 min in an ultrasonic bath prior to use. UVdetection was done at 245 nm. The samples were injected by the aid of a 25 μL Hamilton® analytical syringe. |
| |
| Materials and Reagents |
| |
| Samples |
| |
| Pure sample: Rosuvastatin calcium; kindly supplied by Chemipharm Parmaceutical industry, Egypt, Its purity was found to be 99.75 ± 1.057 by a direct spectrophotometric method [13]. |
| |
| Pharmaceutical dosage forms: Rosuvast tablets labelled to contain 10 mg/tablet rosuvastatin calcium, batch number 100333A, manufactured by Chemipharm Parmaceutical industry, 6th October, Egypt. Sovikan tablets, labelled to contain 10, 20 mg per tablet rosuvastatin calcium Batch numbers: 003, 001, respectively, manufactured by Hikma Pharma, 6th October, Egypt. |
| |
| All chemicals and reagents were of pure spectroscopic analytical grade. 30% H2O2 , ethyl acetate, concentrated ammonia (specific gravity 0.91) (Adwic , El-Nasr Pharmaceutical Chemicals. Co. Cairo, Egypt). De-ionised water, acetonitrile and methanol (E. Merck, Darmstadt, Germany) were of HPLC grade. |
| |
| Standard solutions: Stock standard solution of Rosuvastatin calcium or its oxidative degradation products (1 mg mL−1) in methanol, for spectrodensitometric method. Rosuvastatin calcium working standard solution or its oxidative degradation products (100 μg mL−1) in methanol, for second derivative (2D), ratio-spectra first derivative (1DD) and HPLC methods. |
| |
| Procedures |
| |
| Preparation of oxidative degraded sample: The drug (50 mg) was weighed in a conical flask, dissolved in 20 ml methanol, 5 ml 30% (v/v) hydrogen peroxide was added and the solution was subjected to reflux at 100°C for three hours. The degradation products were separated on preparative TLC plates using a mixture of ethyl acetate: methanol: ammonia (7: 3: 0.01 by volume) as a developing solvent [11]. |
| |
| Second-derivative (2D) method: Two aliquots equivalent to 200 μg of rosuvastatin calcium and 200 μg of mixed degradates working standard solutions (each, 100 μg mL−1) were, separately, transferred into two 10 mL volumetric flasks. The volume was completed with methanol. The zero-order and the second derivative spectra of the prepared solutions were recorded. |
| |
| Linearity: Portions equivalent to 50-300 μg of rosuvastatin calcium working standard solution (100 μg mL−1) were separately transferred to a series of 10 mL volumetric flasks. Each flask was completed to volume with methanol to reach the concentration range of 5-30 μg mL−1. The amplitudes of the second-derivative peaks were measured at 243.6 nm with Δλ= 4 nm and a scaling factor = 100. Calibration graphs were constructed by plotting ΔA/Δλ versus concentration. The regression equations were then computed for the studied drug at the specified wavelength and used for determination of unknown samples containing rosuvastatin calcium. |
| |
| Assay of laboratory-prepared mixtures: Aliquots equivalent to 240-60 μg were accurately transferred from rosuvastatin calcium working standard solution (100 μg mL−1) into a series of 10-ml volumetric flasks. To the previous solutions aliquots equivalent to 60-240 μg of oxidative degradation products (100 μg mL−1) were added. The volumes were completed with methanol and mixed thoroughly. Record the zero order spectra of the prepared mixtures then obtain their second derivative spectra at 243.6 nm. The concentration of rosuvastatin calcium was calculated from its regression equation. |
| |
| Ratio-spectra 1st derivative (1DD) spectrophotometric method |
| |
| Linearity: Standard serial concentrations in the range of 50-350 μg of rosuvastatin calcium working standard solution (100 μg mL−1) were separately transferred to a series of 10 mL volumetric flasks. Aliquot equivalent to 200 μg of the degradates working standard solution (100 μg mL−1) was transferred into a 10 mL volumetric flask and the volume was completed with methanol to be used as a divisor. The spectra of the prepared standard solutions were scanned (200-400 nm) and stored into the PC. The stored spectra of rosuvastatin calcium were divided (amplitude at each wavelength) by the spectrum of 20 μg mL−1 of the degradation product. The first-derivative of the ratio spectra (1DD) with Δλ= 4 nm and a scaling factor = 10 was obtained. The amplitudes of the first-derivative peaks of rosuvastatin calcium were measured at 240 nm. Calibration graphs were constructed relating the peak amplitudes of (1DD) to the corresponding concentrations. The regression equations were then computed for the studied drug at the two specified wavelengths and used for determination of unknown samples containing rosuvastatin calcium. |
| |
| Assay of laboratory-prepared mixtures: Aliquots equivalent to 240-60 μg were accurately transferred from rosuvastatin calcium working standard solution (100 μg mL−1) into a series of 10-ml volumetric flasks. To the previous solutions aliquots equivalent to 60- 240 μg of oxidative degradation products (100 μg mL−1) were added. The volumes were completed with methanol and mixed thoroughly. The 1DD values were recorded at 240 nm. The concentration of rosuvastatin calcium was calculated from its regression equation. |
| |
| Spectrodensitometric method |
| |
| Linearity: Aliquots equivalent to (0.6, 1, 1.4, … 3.4 μL) of rosuvastatin calcium standard stock solution (1000 μg mL−1) were spotted using Camag Linomat autosampler with microsyringe (100 μL). Spots were spaced 1.5 cm apart from each other and 2 cm from the bottom edge of the plate. The plate was developed in a chromatographic tank previously saturated for at least 1 h with the developing mobile phase; ethyl acetate: methanol: ammonia (7:3:0.01, v/v/v), by ascending mode. The plate was removed, dried in air and the spots were visualized under UV lamp at 254 nm and scanned at 245 nm. The calibration curve was plotted between the recorded area under the peak and the corresponding concentration, from which the regression equation was calculated. |
| |
| Assay of laboratory-prepared mixtures: Aliquots equivalent to 2.8 - 0.6 mg were accurately transferred from rosuvastatin calcium stock standard solution (1000 μg mL−1) into a series of 10-ml volumetric flasks. To the previous solutions aliquots equivalent to 0.6—2.8 mg of rosuvastatin oxidative degradation products stock standard solution (1000 μg mL−1) were added. The volumes were completed with methanol and mixed thoroughly. Proceed as mentioned under linearity. Calculate the concentrations from the corresponding regression equation. |
| |
| HPLC method |
| |
| Linearity: Accurately measured volumes of rosuvastatin calcium working standard solution (100 μg mL−1) were transferred into 10- mL measuring flasks, diluted to the volume with methanol to get the final concentration range of 10-60 μg mL−1. The samples were then chromatographed using the following chromatographic conditions: Stationary phase; a 250 mm × 4.6 mm, C18 analytical column , i.d. 5 μm, Waters (USA), mobile phase; water : acetonitrile: methanol (40: 40: 20) (v/v/v). The mobile phase was filtered through a 0.45 μm Millipore membrane filter and was degassed for about 15 min in an ultrasonic bath prior to use, flow rate; 1 mL min−1 (∼25 C)], with UV-detection at 245 nm. The samples were filtered also through a 0.45 μm membrane filter, and were injected by the aid of a 25 μL Hamilton® analytical syringe. The relative peak area ratios to that of external standard (30 μg mL−1) were then plotted versus the corresponding concentrations of rosuvastatin calcium to get the calibration graph and to compute the corresponding regression equation. Concentrations of unknown samples of rosuvastatin calcium were determined using the obtained regression equation. |
| |
| Assay of laboratory prepared mixtures: Aliquots equivalent to 500-100 μg mL−1 were accurately transferred from rosuvastatin calcium working standard solution (100 μg mL−1) into a series of 10-ml volumetric flasks. To the previous solutions aliquots equivalent to 100-500 μg mL−1 of oxidative degradation products (100 μg mL−1) were added. The volumes were completed with methanol and mixed thoroughly. Proceed as mentioned under linearity. Calculate the concentrations from the corresponding regression equation. |
| |
| System suitability: Twenty microlitres of the solvent mixture and the working standard solutions were injected. The system suitability parameters, retention time, tailing factor, theoretical plate count (N), height of theoretical plate (HETP), separation of rosuvastatin calcium peak and its degradation products peak (resolution) and column capacity were studied. |
| |
| Assay of pharmaceutical formulations: Twenty tablets were emptied. A portion of the powder equivalent to 100 mg rosuvastatin calcium was accurately weighed into a 100 mL beaker, dissolved in methanol, for densitometric method (4 × 20 mL) and filtered into a 100-mL measuring flask. The volume was completed with the same solvent (1000 μg mL−1). Ten millilitres of this tablet stock solution (1000 μg mL−1) were transferred into a 100 mL measuring flask and diluted to the mark with methanol to get a final concentration of 100 μg mL−1 for 2D , 1DD and HPLC methods, then the procedures under ‘Construction of calibration curves’ for each method were followed. |
| |
| Results and Discussion |
| |
| Degradation of rosuvastatin calcium |
| |
| Many pharmaceutical compounds undergo degradation during storage or even during the different processes of their manufacture. Several chemical or physical factors can lead to the degradation of drugs [14]. Hydrolysis and oxidation are the most famous chemical degradation routes of drugs [15,16]. The main classes of drugs that are subject to degradation are esters, amides and lactams. It was found that rosuvastatin calcium was liable to degradation uponrefluxing in a strong oxidative medium to give two degradates, demonstrated in Scheme.1. In this work, oxidative rosuvastatin calcium degradation products was prepared, separated and their structure identified by mass spectroscopy where rosuvastatin calcium oxidative degradate I showed molecular ion peak at 497.96 m/z, whereas its oxidative degradate II molecular ion at 514.37 m/z , which are equivalent to their molecular weights. Also by applying HPLC method the retention time for rosuvastatin calcium was 3.9 min, 5.1 min for its oxidative degradate II and 10.7 min. for oxidative degradate I. |
| |
|
|
Scheme 1: Suggested scheme for the oxidative degradation of rosuvastatin calcium. |
|
| |
| The present work was conducted for the selective determination of rosuvastatin calcium in the presence of its oxidative degradation products with the application to pharmaceutical dosage forms. |
| |
| |
| TLC-fractionation |
| |
| TLC-monitoring of the drug degradation was done on thin layer plates of silica gel F254 using ethyl acetate: methanol : ammonia (7:3:0.01, v/v/v) as the developing solvent. The developed plates were visualized under short UV-lamp. |
| |
| The degradates (Rf =0.1, Rf =0.75 for oxidative degradates II and I, respectively); could be separated from the intact drug (Rf value = 0.32). |
| |
| Spectrophotometric methods |
| |
| Second-derivative (2D) method: Derivative spectrophotometry is a powerful tool in quantification of mixture of drugs. It can be also used as a stability-indicating method for the analysis of drugs in presence of their degradation products; as it can solve the problem of absorption bands overlapping. A simple, rapid and selective spectrophotometric technique was proposed and applied for the determination of rosuvastatin calcium in the presence of its degradation products, either as raw material or in pharmaceutical formulations. This was done by applying the second derivative (2D) ultraviolet spectrophotometry. The method can solve the problem of spectral bands overlapping between rosuvastatin calcium and its degradates without sample pretreatment or separation steps of the analyzed drug and its degradates. |
| |
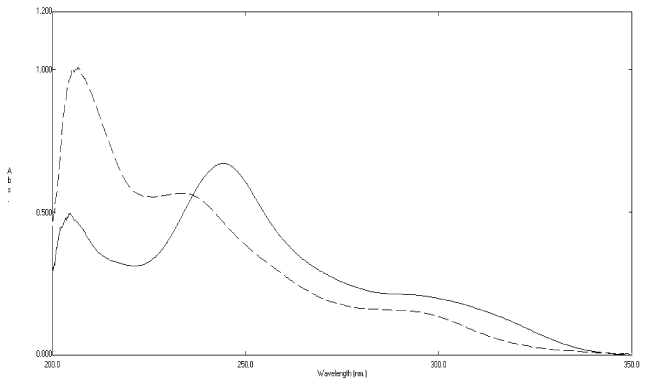
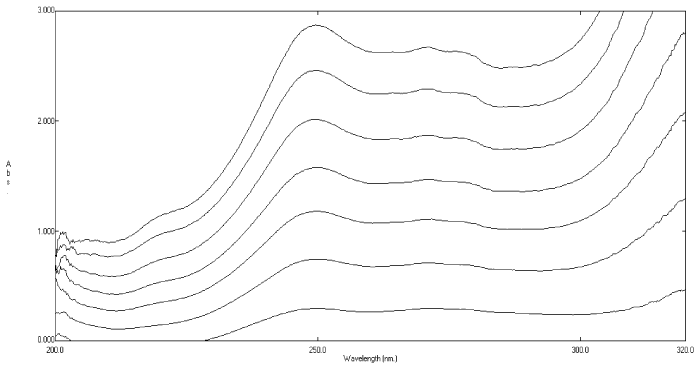
| The zero-order absorption spectra of rosuvastatin calcium and its degradation products showed severe overlap over the entire spectrum of the intact drug, (Figure 2). Therefore, the use of direct absorbance measurements for assaying rosuvastatin calcium in the presence of its degradation products was not possible. |
| |
|
|
Figure 2: Absorption spectra of rosuvastatin calcium 20μg mL-1 (___) and its oxidative degradates 20 μg mL-1 (----) using methanol as a blank. |
|
| |
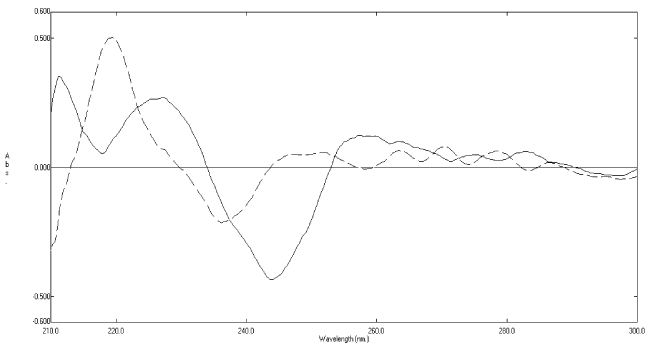
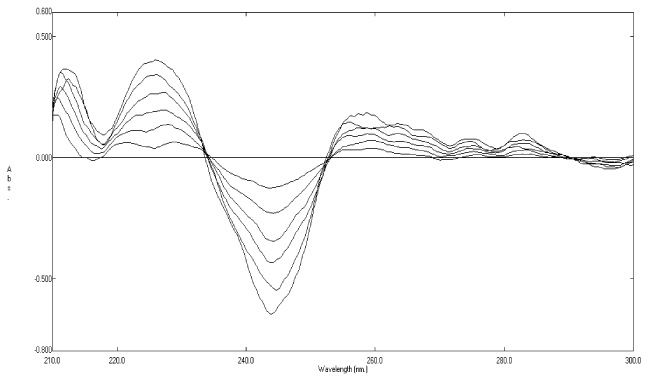
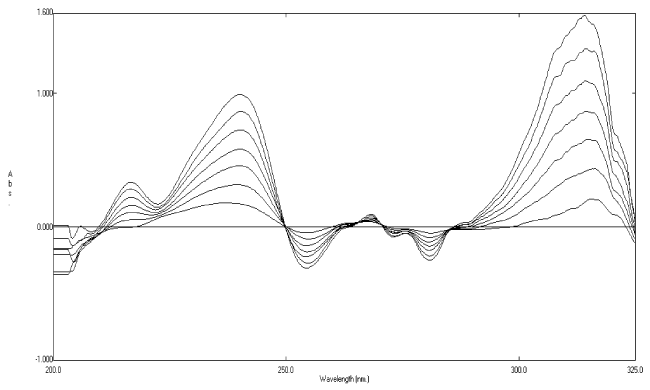
| When the second-derivative spectra (Figure 3) were examined, it was found that rosuvastatin calcium can be determined at 243.6 nm, where its degradates have no contribution (zero crossing). The clear zero crossing of the degradates allows accurate determination of rosuvastatin calcium in presence of of its degradates up to 80%. A linear relationship was obtained in the range of 5-30 μgmL−1 for rosuvastatin calcium (Figure 4). The corresponding regression equations were computed and found to be: 2D = -0.0205C - 0.0241 (r = 0.9996), at 243.6 nm where 2D is the peak amplitude of the secondderivative curve (ΔA/Δλ) at the corresponding wavelength, C is the concentration of rosuvastatin calcium (μgmL−1) and r is the correlation coefficient. |
| |
|
|
Figure 3:Second-derivative absorption spectra of rosuvastatin calcium 20 μg mL-1 (__) and its oxidative degradates 20 μg mL-1 (----) using methanol as a solvent. |
|
| |
|
|
Figure 4: Second-derivative absorption spectra of 5-30 μg mL-1 of rosuvastatin calcium. |
|
| |
| Derivative ratio spectrophotometric method: Ratio-spectra 1st derivative spectrophotometry (1DD) is an analytical technique of good utility which offers background correction and better selectivity than normal spectrophotometry for resolving binary mixtures and some ternary mixtures [17]. |
| |
| The ratio-spectra 1st derivative (1DD) method was suggested to solve this problem. The theory of derivative ratio spectrophotometry, which is based on the use of first (or second) derivatives of the ratio spectra of the mixture and divided (amplitudes at each wavelength) by the absorption spectrum of a standard solution of one of the components, has been applied extensively to the simultaneous determination of substances with overlapping spectra as an economic alternative to HPLC methods [18], and to solve the problem of overlapping absorption spectra of rosuvastatin calcium and its oxidative degradation products. In the present investigation, the careful choice of the divisor and the working wavelength were of great importance as it affected both sensitivity and selectivity; accordingly, different concentrations of the degradation products were tried as divisors. It was found that 20 μg mL−1 was the best, as it produced minimum noise and gave better results in agreement with selectivity. |
| |
| The zero order of the derivative ratio spectra of rosuvastatin calcium and the first-order of the derivative ratio spectra are presented in Figure 5 and 6, respectively. |
| |
|
|
Figure 5: Ratio spectra of rosuvastatin calcium (5-35μg mL-1) using the spectrum of 20 μg mL-1 of oxidative degradates as a divisor. |
|
| |
|
|
Figure 6: First derivative of ratio spectra of rosuvastatin calcium(5-35 μg mL-1) using the spectrum of 20 μg mL-1 of oxidative degradates as a divisor. |
|
| |
| Linear calibration graphs were obtained for rosuvastatin calcium in concentration range of 5-35 μg mL−1 by recording the peak amplitude at 240 nm using the absorption spectra of 20 μg mL−1 oxidative degradation products, as a divisor. The regression equations were computed and found to be: (1DDox240) = 0.0029C + 0.0153 r =0.9996 Where 1DD is the peak amplitude of the first-derivative curve for (rosuvastatin calcium/its degradates), C is the concentration of rosuvastatin calcium in μg mL−1 and r is the correlation coefficient. |
| |
| Spectrodensitometric method |
| |
| TLC densitometry overcomes the problem of overlapping absorption spectra of a mixture of drugs by separating these components on TLC plates and determining each ingredient by scanning the corresponding chromatogram. The TLC-UV densitometric method has the advantage of simultaneously determining the active ingredients in multi-component dosage forms [19]. |
| |
| The proposed procedure is based on the difference in Rf values of rosuvastatin calcium (Rf = 0.32) and its oxidative degradation products (Rf=0.1 , 0.75 for oxidative degradate II and I, respectively. Various developing systems were tried, but complete separation was achieved using ethyl acetate: methanol: ammonia (7:3:0.01, v/v/v). |
| |
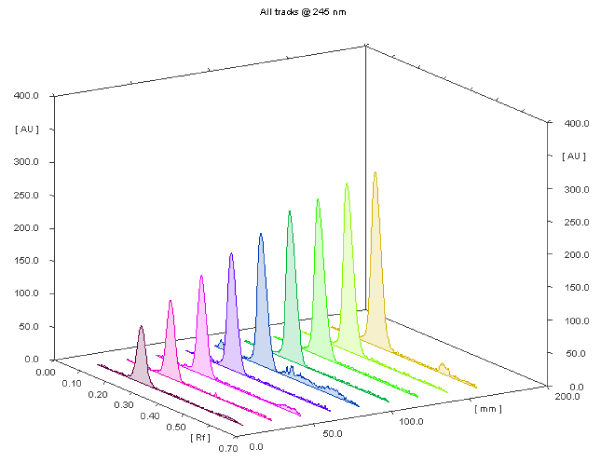
| The linearity was confirmed by plotting the measured peak area versus the corresponding concentrations at 245 nm over a range of 0.6- 3.4 μg / spot, where a linear response was obtained. Scanning profile of different concentrations of rosuvastatin calcium at 245 nm was shown in (Figure 7). The linear regression equation was found to be: A=0.2653C + 0.166 r=0.9996 where A is the integrated area under the peak×10-4, C is the concentration in μg / spot and r is the correlation coefficient. |
| |
|
|
Figure 7: Scanning profile of the TLC chromatogram of rosuvastatin calcium (0.6 -3.4 μg spot-1) at 245 nm. |
|
| |
| HPLC method |
| |
| A simple HPLC method was adopted for the simultaneous determination of rosuvastatin calcium in the presence of its oxidative degradation products without pervious separation. |
| |
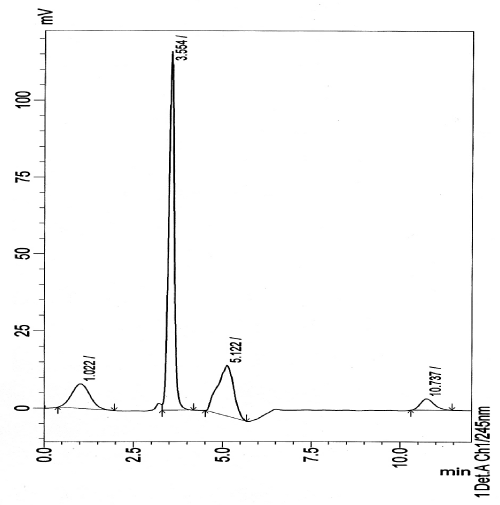
| Different mobile systems were tried, The best resolution was achieved when using a mobile phase consisting of water: acetonitrile: methanol (40: 40: 20, v/v/v) using UV detection at 245 nm to obtain a retention time 3.5 min for rosuvastatin calcium , 5.1 min for its oxidative degradate II and 10.7 min. for oxidative degradate I, (Figure 8). |
| |
|
|
Figure 8: HPLC chromatogram of rosuvastatin calcium (20 μg mL-1 Rt :3.55 min.) its oxidative degradate II (10 μg mL-1, Rt :5.12 min.) and its oxidative degradate I (10 μg mL-1 Rt : 10.74 min.). |
|
| |
| A linear relation was obtained between peak area and the concentration of rosuvastatin calcium in the range of 10-60 μg mL−1. The linear regression equation was found to be: A= 0.033C+0.0054r=0.9998 where A is the relative peak area, C is the concentration in μg mL−1 and r is the correlation coefficient. |
| |
| Stability indication |
| |
| To assess the stability-indicating efficiency of the proposed methods, the degradation products of rosuvastatin calcium were mixed with its intact sample in different ratios and analyzed by the proposed methods. Table 1 illustrates good selectivity in the determination of rosuvastatin calcium in the presence of up to 80% (w/w) of its degradates in the second-derivative and the derivative-ratio spectrophotometric methods, up to 82% (w/w) by the densitometric method and up to 83% (w/w) by HPLC method. |
| |
|
|
Table 1: Determination of rosuvastatin calcium in laboratory prepared mixtures by the proposed Methods. |
|
| |
| The suggested methods were successfully applied for the determination of rosuvastatin calcium in its pharmaceutical formulations, showing good percentage recoveries. The validity of the suggested methods was further assessed by applying the standard addition technique (Table 2). System suitability tests, which are used to ensure system performance before or during the analysis of drugs, were performed. The obtained values of rosuvastatin calcium and its oxidative degradation products were agreed with the stated reference values, (Table 3). Assay validation was done by repeating the procedures three times on three different days (interday) and three times on different time intervals within the same day (intraday) for the analysis of different concentrations of rosuvastatin calcium, (Table 4). The results show that the methods were accurate, precise and specific. Results of the suggested methods for determination of rosuvastatin calcium were statistically compared with those obtained by applying reported spectrophotometric method [13]. The calculated t- and F-values were found to be less than the corresponding theoretical ones, confirming good accuracy and excellent precision (Table 5). |
| |
|
|
Table 2: Determination of rosuvastatin calcium in pharmaceutical formulations by the proposed methods and results of application of standard addition technique. |
|
| |
|
|
Table 3: System suitability parameters of the elaborated HPLC method for the analysis of rosuvastatin calcium in the presence of its oxidative degradation products. |
|
| |
|
|
Table 4: Validation of the results obtained by applying the suggested methods for the determination of rosuvastatin calcium. |
|
| |
|
|
Table 5: Statistical analysis of the results obtained by applying the proposed methods and a reported spectrophotometric method for the analysis of pure rosuvastatin calcium. |
|
| |
| Conclusion |
| |
| Four methods, 2D, 1DD, TLC and HPLC were developed for the determination of rosuvastatin calcium in the presence of its oxidative degradation products. The methods provide simple, accurate, rapid and reproducible quantitative analysis of rosuvastatin calcium in bulk powder, laboratory-prepared mixtures and dosage forms. |
| |
| The 2D, 1DD methods have the advantages of being more economical, rapid and environmentally secure than the other methods. The TLC method was found to be more sensitive than the 2D, 1DD methods. The proposed HPLC method gives a good resolution between rosuvastatin calcium and its oxidative degradation products within a short time. These methods can be used as stability-indicating procedures in quality control laboratories where economy and time are essential. |
| |
| |
| References |
| |
- Mori Y, Kuriyama G, Tanaka T, Tajima N (2009) Usefulness of aggressive lipid-lowering therapy with rosuvastatin in hypercholesterolemic patients with concomitant type 2 diabetes. Endocrine.36:412-418.
- Jones P, Davidson M, Stein E, Bays H, McKenney J, et al. (2003) Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses. Am J Cardiol. 92:152-160.
- Bergmana E, Forsell P, Tevell A, Perssona E, Hedelandc M, et al.(2006) Biliary secretion of rosuvastatin and bile acids in humans during the absorption phase. Eur J Pharm Sci. 29:205-214.
- Mehta T, Patel A, Kulkarni G, Subbaiah G (2005) Determination of rosuvastatin in the presence of its degradation products by a stability-indicating LC method. J AOAC Int. 88:1142-1147.
- Hull C, Penman A, Smith C, Martin P (2002) Quantification of rosuvastatin in human plasma by automated solid-phase extraction using tandem mass spectrometric detection. J Chromatogr B Analyt Technol Biomed Life Sci.722: 219-228.
- Sane R, Kamat S, Menon S, Inamdar S, Mote M (2005) J PC-Modern TLC 18:194-198.
- Hull C, Martin P, Warwick M, Thomas E (2004) Quantification of the N-desmethyl metabolite of rosuvastatin in human plasma by automated SPE followed by HPLC with tandem MS detection. J. Pharm Biomed Anal. 35:609-14.
- Trivedi R, Kallem R, Mullani R, Srinivas N (2005) Simultaneous determination of rosuvastatin and fenofibric acid in human plasma by LC-MS/MS with electrospray ionization: assay development, validation and application to a clinical study. J Pharm Biomed Anal. 39:661-669.
- Uyar B, Celebier M, Altinoz S (2007) Spectrometric determination of rosuvastatin calcium in tablets. Pharmazie. 62:411-3.
- I.Süslü, M. Çelebier, S. Alt nöz. J. Chromatogr. (2007); 66: 65-72.
- Amr B, Nadia M, Abd El-Aziz A, Nesrine L (2011) Selective determination of bambuterol hydrochloride in the presence of its active metabolite terbutaline. Int. J. of Pharmacy and Pharmaceutical Sciences.
- Food and drug administration,HHS (2003) International Conference on Hormonisation (ICH): Guidance on QLD bracketing and matrixing designs for stability testing of new drug substances and products. U.S. department of health and human services.
- Gupta A, Mishra P, Shah K (2009) E-J Chem. 6: 89- 92.
- Henry M, Charles O, Wait R (1963) Physical and Technical Pharmacy, 621-644, Mc Graw-Hill Book Co., Inc., New York, Toronto, London.
- A. Florence, D. Attwood (1998) Physicochemical Principles of Pharmacy. (3rd Ed.), London: Macmillan Press.
- G. Banker, C. Rhodes (2002) Modern pharmaceutics. (4th Ed.), Marcel Dekker Inc.
- Nevado J, Flores J, Cabanillas C, Llerena M, Salcedo A (1998) Resolution of ternary mixtures of Tartrazine, Sunset yellow and Ponceau 4R by derivative spectrophotometric ratio spectrum-zero crossing method in commercial foods. Talanta.46:933-942.
- Salinas F, Berzas J, Espinosa M(1990) Resolution of ternary mixtures of Tartrazine, Sunset yellow and Ponceau 4R by derivative spectrophotometric ratio spectrum-zero crossing method in commercial foods. Talanta 37:347-351.
- Bebawy LI, El Kousy N (1999) Determination of some antihistaminic drugs by atomic absorption spectrometry and colorimetric methods. J Pharm Biomed Anal. 20, 663-670.
|
| |
| |