| Research Article |
Open Access |
|
| Nazish Fatima*, Mithlesh Agrawal, Indu Shukla and Parvez Anwar khan |
| Department of Microbiology, J.N. Medical College, AMU, Aligarh, India |
| *Corresponding authors: |
Nazish Fatima
Department of Microbiology
J.N. Medical College, AMU
Aligarh, India
E-mail: medicalmicrobiologyjnmc@gmail.com |
|
| |
| Received August 15, 2012; Published September 24, 2012 |
| |
| Citation: Fatima N, Agrawal M, Shukla I, Khan PA (2012) Characterization of Uropathogenic E. coli in relation to virulence Factors. 1:342. doi:10.4172/scientificreports.342 |
| |
| Copyright: © 2012 Fatima N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| 250 urinary isolates of E. coli were studied for the presence of various virulence markers i.e., haemolysin, haemagglutination of human O erythrocytes, cell surface hydrophobicity, adhesion and O serotype prevalence. Fifty stool isolates of E. coli were taken as controls. Of the 250 isolates, 75 exhibited haemolysin (p<0.001), 75 exhibited mannose resistant haemagglutination (p<0.001), 120 (p<0.01) showed adhesion, 30 (not significant) were resistant to bactericidal activity of serum, 55 showed cell surface hydrophobicity (p<0.05).Common serotypes encountered in this study were O25, O20, O7, O50, O4, O6, O26 and O51. Presence of multiple virulence markers also noticed. We believe that the methods of detection of the above mentioned virulence markers are reasonably easy and screening them in clinical microbiology laboratories is a worthwhile exercise. |
| |
| Introduction |
| |
| Urinary tract infections (UTIs) are one of the most common infections encountered in clinical practice [1] mainly being associated with different members of the family Enterobacteriaceae and among them Escherichia coli (E. coli) is the most predominant pathogen [2]. Certain serotypes of E. coli are consistently associated with uropathogenicity and are designated as uropathogenic E. coli (UPEC) [3]. Uropathogenic strains account for 90% of all UTIs among ambulatory patients and up to 50% of all nosocomial UTIs [4]. Concept of uropathogenic bacteria refer to certain strains that are selected from faecal flora, not by chance or based on prevalence but because of specific virulence factors. Uropathogenic E. coli strains are believed to display a variety of virulence properties that help them to colonize host mucosal surface and circumvent host defense to allow invasion of the normally sterile urinary tract [2]. Since 1970s, an array of virulence factors have been proposed as virulence markers for uropathogenic isolates of E. coli. These include capsular K antigen, somatic O antigen, adherence, haemagglutination of erythrocytes, haemolysin, resistance to the bactericidal activity of serum, phagocytosis, cell surface hydrophobicity, expression of siderophore aerobactin, production of colicin V and cytotoxic necrotizing factor. These UPEC isolates express chromosomally encoded virulence markers and these markers of UPEC are expressed with different frequencies in different disease states ranging from asymptomatic bacteriuria to chronic pyelonephritis. |
| |
| This study was undertaken to determine the prevalence of virulence factors (haemolysin, serum resistance, haemagglutination of human erythrocyte and effect of D-mannose on haemagglutination, adhesion and cell surface hydrophobicity) in urinary and faecal isolates of E. coli obtained from clinical samples in a North Indian tertiary hospital and to know the distribution of different O serotypes of E. coli isolates. |
| |
| Materials and Methods |
| |
| The study was conducted on a total of 250 strains of E. coli obtained from same number of symptomatic cases of UTI whose urine samples were processed in Department of Microbiology, JN Medical College & Hospital, Aligarh, India for routine culture and susceptibility. A total of 50 isolates of E. coli from stool samples of apparently healthy individuals were included as controls. |
| |
| The urine samples were inoculated on 5% sheep blood agar and MacConkey (MA) agar and then incubated at 37°C for 24 hrs. Culture plates with a growth of single morphotype of E. coli having counts ≥ 105 CFU/ml were considered significant. E. coli was identified and characterized on the basis of their colony characters, morphology and biochemical reactions as per the standard methods [5]. |
| |
| Studies determining virulence factors |
| |
| All the isolates from urine and stool specimens were tested for the virulence factors as described below: |
| |
| Haemolysin: The isolates to be tested were subcultured onto 5% sheep blood agar and incubated overnight at 37°C. Haemolysin was detected by determining a clear haemolytic zone (β-haemolysis) around each colony [6]. |
| |
| Haemagglutination of Human group O erythrocytes: The test was carried out on Venereal Disease Research Laboratory (VDRL) slides. One drop (100 μl) of bacterial suspension was mixed with one drop of erythrocytes and one drop of phosphate-buffered saline (PBS) with and without 3% mannose on a VDRL slide. The slide was rotated for five minutes at room temperature and the presence or absence of macroscopic haemagglutination was noted. Haemagglutination was considered to be mannose resistant (MRHA) when it occurred in the presence of D-mannose and mannose sensitive (MSHA), when it was inhibited by the presence of D-mannose [7]. |
| |
| Cell surface hydrophobicity (CSH): Different molar concentrations of ammonium sulphate including 1 M, 1.4 M and 2 M were prepared. Forty microliter of 0.2 M PBS (pH 6.8) was taken in first column of VDRL slide. Forty microliter of 1 M, 1.4 M and 2 M concentration of ammonium sulphate were taken in each well of other columns of the VDRL slide. Forty microliter of E. coli suspension (5x109 cfu/ml) was added to each of these wells. The clumps formed in different molar concentration of ammonium sulphate were observed microscopically under 100x and 400x magnification. Strains were considered hydrophobic if aggregation in a concentration of 1.4 M was seen [7]. |
| |
| Serum bactericidal assay (SBA): E. coli cell suspensions in Hank’s Balanced Salt Solution (HBSS) were prepared (2.5×104 cfu/ml) in the microtitre plate and to each well of a microtitration plate, 0.05 ml of 100% serum was added. The control well contained 0.05 ml of HBSS instead of serum. Then from each well samples (10 μl) were withdrawn after incubation for 60, 120 and 180 min at 37°C and were spread on blood agar plates. The plates were incubated for 18 hrs at 37°C and viable counts were determined. Susceptibility of bacteria and serum bactericidal activity was expressed as the percentage of bacteria surviving after 180 minutes in relation to the original count of bacteria determined at 0 minutes in the controls. Strains were termed serum sensitive if the viable count dropped to 1% of the initial value and resistant if more than 90% of organisms survived after 180 minutes [7]. |
| |
| Adhesion: A mixture of 108 bacteria and 105 epithelial cells was incubated in 1 ml of PBS for 1 hr at 37°C with repeated shaking. Unattached bacteria were removed by repeated washing in PBS. A drop of trypan blue was added to the mixture and bacteria attached to one epithelial cells estimated. The degree of adherence was taken as Weak 1+, moderate 2+, strong 3+; 2+ or more was taken as positive adherence [8]. |
| |
| Serotyping: Isolates were serotyped at Central Research Institute, Kasauli (National Salmonella & Escherichia Centre), India. |
| |
| Antibiotic Susceptibility Testing: Antibiotic Susceptibility Testing was done according to the method of Bauer et al. The antibiotic discs (Hi Media, India) used in the present study are shown in Table 2. |
| |
| Results and Discussion |
| |
| Virulence markers |
| |
| Of the 250 urinary isolates from patients presenting with UTI cases, 75 (30%) showed haemolytic activity among 50 isolates from healthy volunteers, only 2 (4%) showed haemolysis. The difference between cases and controls for haemolysin production was highly significant (p<0.001). Of those 250 isolates, 120 (48%) isolates showed Haemagglutination (HA) of group O RBCs and 130 (52%) were nonhaemagglutinating. Out of 120 isolates tested strains positive for HA, 75 (30.0%) were MRHA positive and 45 (18.0%) were MSHA. Where as in the control group 3 isolates out of 50 showed HA and all the 3 showed MRHA. The difference in MRHA between cases and controls was highly significant (p<0.001) (Table 1). Of the 250 isolates, 120 (48%) isolates from UTI patients isolates from and 5 (10%) isolates from controls showed adhesion to uroepithelial cells. The difference between cases and controls for adherence to epithelial cells was found highly significant (p<0.01) (Table 1). |
| |
|
|
Table 1: Virulence markers detected in Uropathogenic E. coli (UPEC) from cases and E. coli isolates from controls. |
|
| |
| Resistance to bacterial effect of serum was seen in 30 (12%) isolates of E. coli from patients and in only 2 (4%) isolates in the control group and this difference was not found to be statistically significant (Table 1). A total of 55 (22%) isolates among 250 cases and 4 (8%) isolates among 50 controls showed cell surface hydrophobicity. The difference between cases and controls for cell surface hydrophobicity was found statistically significant (p<0.05) (Table 1). It was observed that among the different virulence markers tested, adhesion and haemagglutination were found in highest number of isolates from UTI patients. In the control group, adhesion and cell surface hydrophobicity were the two most commonly associated markers. |
| |
| |
| Antibiotic sensitivity |
| |
| Antibiotic sensitivity pattern of uropathogenic Escherichia showed maximum resistance to nalidixic acid (84%) which is an antibiotic specially used in the cases of UTI, high prevalence of resistance to this antibiotic is really a matter of concern. Least resistance (40%) was seen with Netilimicin. Majority of isolates were multidrug resistant (>3 drugs) and none of the strains were found to be sensitive to all the antibiotics tested (Table 2). |
| |
|
|
Table 2: Antibiotic sensitivity pattern of Uropathogenic Escherichia coli strains. |
|
| |
| Serotyping |
| |
| Out of total 250 isolates of E. coli in the present study, 195 could be serotyped and of which 181 were found typeable, 9 were non-typeable and 5 were reported as rough strains. Of the 181 typeable E. coli strains, majority belonged to serotypes O2, O4, O6, O12, O18, O20, O25, O50, O51, O71, and O101. The most common serotypes among the E coli isolates from the patients were O25 (25 strains) followed by O20 (18 strains), O2 and O50 (12 strains each), O4 (11 strains), O6 (9 strains) O26 and O51 (8 strains each), O101 (7 strains) and O12, O18 and O75 (6 strains each). |
| |
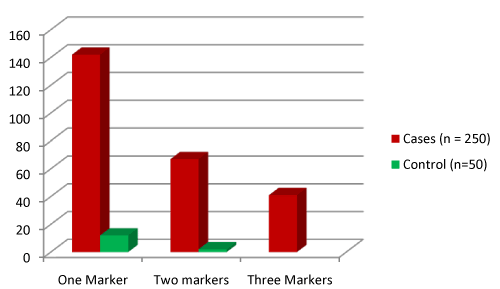
| One hundred and forty two cases out of total 250 exhibited one virulence marker, 67 showed two virulence markers and 41 cases showed three virulence markers. Among 142 one marker positive cases, majority of UPEC (99) produced adhesion. Among 67 two marker positive cases, notably 24 UPEC produced lytic+MRHA, 19 UPEC produced MRHA+CSH and other combinations of virulence markers were also seen. Among 41 three marker positive cases, 22 UPEC produced lytic+MRHA+CSH and 10 UPEC produced lytic+MRHA+SR. Most of the faecal strains from controls exhibited no virulence associated properties. |
| |
| Considering the high degree of morbidity and mortality of UTIs the subject of uropathogenic E. coli is receiving increasing attention. UPEC is reported to express several virulence factors. In our study, the virulence factors studied were significantly high (p<0.01) in cases as opposed to controls. We inferred that UPEC strains are definitively associated with the aetio-pathogenesis of UTI. Our findings correlated well with the findings of most other workers who also found significantly higher values of virulence factors in UPEC compared to faecal isolates of E. coli from the controls [6,9-12]. Haemolytic activity of E. coli is related to haemolysin production. Four different types of haemolysins have been described. Most important being α and β haemolysins [13]. Raksha et al. also found significant difference (p<0.001) in haemolysin production between cases and controls [6]. Cytotoxicity and stimulation of growth of bacterium by making iron available appear to be the most likely mechanisms by which haemolysin may function to increase the virulence of E. coli. |
| |
| Agglutination of human erythrocytes by E. coli strain is an indirect evidence of the presence of fimbriae on that strain [14]. MRHA positive strains can be considered as UPEC most likely having P fimbriae [15]. E. coli strains associated with severe form of UTIs are reported to exhibit mannose resistant haemagglutination [14,16]. In the present study, difference between cases and controls was highly significant (p<0.001). This was similar to studies conducted by many workers who also found significant difference in MRHA between cases and controls [6,15]. |
| |
| A number of studies have suggested a possible correlation between the virulence of an organism and the ability of that organism to adhere to the surface of an organ and play an important role in the pathogenesis of urinary tract infection [17,18]. In the present study the difference in the ability to attach to uroepithelial cells was found to be highly significant (p<0.001). Thus, indicating that adherence has an important role in the pathogenecity of UTIs. |
| |
| Bacteria are killed by normal human serum through lytic activity of the alternative complement pathway [19]. Bacterial resistance to killing by serum results due to capsular polysaccharides and surface proteins. We observed that although more E. coli isolates from cases were resistant to anti bacterial activity of serum as compared to controls, the difference was not statistically significant. |
| |
| The role of CSH in mediating bacterial adherence to mammalian cells was conceived by Mudd and Mudd [20]. Hydrophobicity is a recently described novel virulence mechanism in E. coli and it has a role in mediating bacterial adherence to mammalian cells. Crystalline surface layer ‘S’ present on both Gram positive and Gram negative organisms play an important role in this pathogenesis [21]. We found significant difference in cell surface hydrophobicity between E. coli isolates from cases with UTI and controls (p<0.05). Contradictory to our findings, Raksha et al. found no significant difference in CSH between cases and controls [6]. |
| |
| Uropathogenic isolates of E. coli belonging to certain serogroups possess specific virulence factors which enhance the ability to cause infection. In most studies E. coli isolates from UTI belonged to ‘Uropathogenic’ serotypes O1, O2, O4, O6, O7, O8, O16, O18, O25 and O75 [22]. The serotypes encountered in this study were among the conventional serotypes of uropathogenic E. coli and many late serotypes were also involved i.e. O25, O20, O2, O50, O4, O6 and O51. Serotypes O4 and O6 have been reported to be the most frequently isolated from urine in many studies [3,23] but in our study serotypes O25 and O20 were the predominant types found. Haemolytic E. coli strains were found to occur with greater frequency in the serotypes O2, O4, O6, O20 and O25. MRHA positive UPEC were found to occur with greater frequency in serotypes O2, O20, O25 and O50 (24) but in our study serotypes O25 and O20 were found to be predominant. |
| |
| Haemolysin production and the capacity to cause MRHA emerged as the most important virulence factors. Haemolysin, especially α-haemolysin is strongly pro-inflammatory leading to secretion of IL-6 and chemotaxins which sets the pace for pathogenesis of renal disease. The capacity to cause MRHA is due to various adhesions mainly P fimbriae, P associated fimbriae and FIC fimbriae seen in pyelonephritis cases. These adhere to fibronectin on uroepithelial cells contributing to persistence. |
| |
| The presence of multiple virulence factors was also noticed (Figure 1). The occurrence of multiple virulence markers in UPEC strains strengthens the concepts association of UPEC in uropathogenecity of urinary tract. It was interesting to note that UPEC with multiple virulence factors were significantly more in cases than in controls. |
| |
|
|
Figure 1: Occurrence of Single/multiple virulence markers in UPEC strains among cases and E. coli isolates from controls. |
|
| |
| Conclusions |
| |
| In this study we concluded that UPEC strains are definitely associated with the aetiopathogenic of UTI. E.coli strains that cause UTI typically produce specific virulence determinants. Such as haemolysin, haemagglutination of human group O erythrocytes, cell surface hydrophobicity, serum resistance and adhesion to uroepithelial cells. We believe that the methods of detection of the above mentioned virulence markers are reasonably easy and screening them in a clinical microbiology laboratories is a worthwhile exercise. |
| |
| Acknowledgement |
| |
| We are thankful to Central Research Institute, Kasauli (National Salmonella & Escherichia Centre), India for the serotyping of the E. coli isolates. |
| |
| |
| References |
| |
- Sharma S (1997) Current understanding of pathogenic mechanisms in UTIs. Ann Natl Acad Med Sci 33: 31-38.
- Mobley HLT (2000) Virulence of the two primary uropathogen. ASM News 66: 403-410.
- Fule RP, Menon S, Saoji AM (1990) Antibiotic resistance, haemagglutination type & haemolysin production in relation to serogroups of uropathogenic Escherichia coli. Indian J Med Res 91: 270-272.
- Steadman R, Topley N (1998) The virulence of Escherichia coli in urinary tract, In: Urinary tract infections. Chapman and Hall publication, London.
- Collee JG, Mackie TJ, McCartney JE (1996) practical medical microbiology. (14thedn), Churchill Livingstone, New York.
- Raksha R, Shrinivasa H, Mawcaden RS (2003) Occurrence and characterization of uropathogenic Escherichia coli in urinary tract infection. Ind J Med Microbiol 21: 102-107.
- Siegfried L, Kmetová M, Puzová H, Molokácová M, Filka J (1994) Virulence associated factors in Escherichia coli strains isolated from children with urinary tract infections. J Med Microbiol 41: 127-132.
- Eden CS, Hanson LA, Jodal U, Lindberg U, Akerlund AS (1976) Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet 1: 490-492.
- Bonacorsi SP, Clermont O, Tinsley C, Le Gall I, Beaudoin JC,et al. (2000) Identification of regions of the Escherichia coli chromosome specific for neonatal meningitis-associated strains. Infect Immun 68: 2096-2101.
- Hooton TM (2000) Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother 46: 1-7.
- Rizvi M, Kumar S (2003) Serum resistance of Escherichia coli strains causing urinary tract infection and diarrhoea in relation to alpha haemolysin production and O type. Indian J Pathol Microbiol 46: 504-506.
- Mulvey MA (2002) Adhesion and entry of uropathogenic Escherichia coli. Cell Microbiol 4: 257-271.
- Van den Bosch JF, Postma P, Koopman PA, de Graaff J, MacLaren DM, et al. (1982) Virulence of urinary and faecal Escherichia coli in relation to serotype, haemolysis and haemagglutination. J Hyg (Lond) 88: 567-577.
- Kallenius G, Mollby R, Winberg J (1980) In vitro adhesion of uropathogenic Escherichia coli to human periurethral cells. Infect Immun 28: 972-980.
- Johnson JR (1991) Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev 4: 80-128.
- Vosti KL (1979) Relationship of hemagglutination to other biological properties of serologically classified isolates of Escherichia coli. Infect Immun 25: 507-512.
- Schaeffer AJ, Amundsen SK, Schmidt LN (1979) Adherence of Escherichia coli to human urinary tract epithelial cells. Infect Immun 24: 753-759.
- Virkola R, Westerlund B, Holthofer H, Parkkinen J, Kekomaki M, et al. (1988) Binding characteristics of Escherichia coli adhesins in human urinary bladder. Infect Immun 56: 2615-2622.
- Taylor PW (1983) Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev 47: 46-83.
- Mudd S, Mudd EB (1924) The penetration of bacteria through capillary spaces IV. A kinetic mechanism in interfaces. J Exp Med 40: 633-645.
- Sleytr UB, Messner P (1983) Crystalline surface layers on bacteria. Annu Rev Microbiol 37: 311-339.
- Vosti KL, Goldberg LN, Monto AS, Rantz LA (19664) Host parasite interaction in patients with infections due to Escherichia coli I. The serogrouping of E. coli from intestinal and extraintestinal sources. J Clin Invest 43: 2377-2378.
- Bhalla, Aggarwal P (1989) Urinary Escherichia coli- prevalent serotypes, antimicrobial susceptibility and virulence factors. Indian J Med Micribiol 7: 88-90.
- Shrikhande SN, Chande CA, Pathak AA (1999) Virulence factors in Uropathogenic E. coli. Indian J Pathol Microbiol 42: 321-325.
|
| |
| |