| Research Article |
Open Access |
|
| Gulab Chand Shah*, Mahavir Yadav and Archana Tiwari |
| School of Biotechnology, Rajiv Gandhi Proudyogiki Vishwavidyalaya, Bhopal, Madhya Pradesh (University of Technology of Madhya Pradesh) Airport Bypass Road, Gandhi Nagar, Bhopal-462 033, India |
| *Corresponding authors: |
Gulab Chand Shah
School of Biotechnology
Rajiv Gandhi Proudyogiki Vishwavidyalaya
(University of Technology of Madhya Pradesh) Airport Bypass Road Gandhi Nagar, Bhopal-462 033, India
Tel: 0755-2678803(Office)
Fax: 0755-2742002-3
E-mail: gulab777@gmail.com |
|
| |
| Received September 12, 2012; Published September 28, 2012 |
| |
| Citation: Shah GC, Yadav M, Tiwari A (2012) Analysis and Characterization of Algal Oil by using Different Chromatographic Techniques for the Higher Production of Biodiesel from Scenedesmus Dimorphus Algal Species. 1:350. doi:10.4172/scientificreports.350 |
| |
| Copyright: © 2012 Shah GC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Algae are the fastest-growing plants on the earth, this study demonstrates the culturing of algal strain on MBM, CHU13 media and production of algal biodiesel from Scenedesmus dimorphus, Biodiesel is an alternative fuel for conventional diesel that is made from natural plant oils, animal fats, and waste cooking oils. This paper discusses the economics of producing oil from algae by soxhlet, ultrasonic wave, and expeller method grown in open ponds. Microalgae have been identified as a potential biodiesel feedstock due to their high lipid productivity and the process conditions are milder than those required for pyrolysis and prevent the formation of by-products. Algae are very important as a biomass source. Analysis of algal oil by GC, TLC, and paper chromatography, algae will someday be competitive as a source for biofuel. Algae can be grown almost anywhere, even on sewage or salt water, and does not require fertile land or food crops, and processing requires less energy than the algae provides. Algae can be a replacement for oil based fuels, one that is more effective and has no disadvantages. About 50% of algal oil converted to biodiesel by trans-esterification process. This microalgal oil can be used to make biofuels for bus, and other vehicles. |
| |
| Keywords |
| |
| Microalgae; Biofuels; Lipid; Biomass; Glycerol; Transesterification |
| |
| Abbreviation |
| |
| GHC: Green House Gas; ASTM: American Society of Testing Materials; FAME: Fatty Acid Alkyl Ester, TAGs: Try Acyl Glycerol’s; MFC: Microbial Fuel Cell; MAO: Microalgae oil; TG: Tri Glycerides; PAHs: Polycyclic Aromatic Hydrocarbons; MBM: Modified Basal Medium; GC: Gas Chromatography; TLC: Thin Layer Chromatography |
| |
| Introduction |
| |
| A constant rising worldwide demand of motor and power generation fuels, together with environmental concerns in terms of Green House Gases (GHG), has motivated the scientists and technologists to think about various alternate sources of energy [1]. With the increasing amount of waste originating from human activities comes the negative impact on the environment and in particular the water quality. Waste streams, which are rich in car-bon, nitrogen and other minerals, have potential for use as a substrate for microalgae cultivation [2,3]. Biodiesel is derived from the trans-esterification of mono-, di- and triacylglycerides (TAGs) and the esterification of free fatty acids (FFAs) that occur naturally in biological lipids, such as animal fats and plant oils. As a result, biodiesel has the potential to be a carbon neutral fuel [4-6]. |
| |
| Although industrial-scale facilities for biodiesel production from microalgae have not been built, there has been substantial research performed on the feasibility, design and requirements for such a production system. A near-complete design for a large (400 ha) production system to produce biodiesel from algae is in [7] as well as recommendations on exactly where in Australia such facilities could be situated, whilst [8] contains additional information on algal production, including economic considerations and identifies several additional pieces of equipment necessary for production not outlined in [7]. |
| |
| It would be valuable to be able to extract and convert triglycerides in microalgae into biodiesel in a single step, bypassing the use of large quantities of organic solvents. Such in situ or direct trans-esterification approaches have been used as an analytical technique to prepare FAMEs for the determination of the fatty acid composition of lipid containing tissues [9-11]. |
| |
| Higher biomass productivity and lower production costs will also encourage production in the tropics. Therefore, bio-fuels have the potential to provide opportunities for economic development and improved energy access for developing countries. However, the negative impacts of increased global demand for bio-fuels are of increasing concern, and include direct and indirect land use change, competition with food production, and land tenure conflicts [12-18]. |
| |
| Materials and Methods |
| |
| Materials |
| |
| The proposed study was done at School of Biotechnology, Rajiv Gandhi Proudyogiki Vishwavidyalaya, Bhopal, Madhya Pradesh. All the chemicals and glass wares used in the proposed study were procured from Himedia and VK traders respectively. All the techniques and protocols used in the proposed study were standardized according to the literature available. |
| |
| Algae sample collection: Collect algae sample from various (Upper lake, Colar dam, pond near by bhanpur dumping sites, Narmada hosangabad) places. |
| |
| Methods |
| |
| Identification of suitable strain |
| |
| Media for Scandasmus Dimorphus algae growth: There were two types of media used in desire algal culture. Elemental composition are following (Tables 1 and 2). |
| |
|
|
Table 1: Composition of Modified Basal Medium. |
|
| |
|
|
Table 2: Composition of Modified CHU 13 Medium. |
|
| |
| Media preparation |
| |
| • Take above medium and make it (100 ml). |
| |
| • Autoclave it at 121°C, 15 lbs pressure, for 15 min. |
| |
| • Inoculate algal sample into four (100 ml) different conical flasks. |
| |
| • Incubate it at normal RT, for 24 hour. |
| |
| • Observe growth into different conical flasks. |
| |
| • Transfer it into 1000 ml media containing flask. |
| |
| • Monitor growth of algae (day/day). |
| |
| • After 10-15 days algae will be used to further processing. |
| |
| Algae harvesting [19] |
| |
| Micro-screening |
| |
| • Algae with media |
| |
| • Filter with sieves |
| |
| • Discard excess water. |
| |
| • Use algae for further processing. |
| |
| Centrifugation |
| |
| • Medium containing algae. |
| |
| • Take 30ml centrifuge tube, transfer medium containing algae to the tube. |
| |
| • Centrifuge it with 4000rpm, for 5 minutes, at room temperature. |
| |
| • Discard supernatant and keep pellets for further oil processing. |
| |
| Extraction of oil from algae |
| |
| Expeller press method [20] |
| |
| • Take 500 g of shade dried algae. |
| |
| • Transfer it into expeller machine for extraction of algal oil. |
| |
| • Run the expeller machine. |
| |
| • After some time collect oil from machine. |
| |
| • Algal oil will be further use for biodiesel production. |
| |
| Soxhlet extraction |
| |
| • Take 100 g of dried powder of algae. |
| |
| • Transfer it into soxhlet apparatus. |
| |
| • Add 100 ml hexane solvent, to rapture cell wall of algae. |
| |
| • Run the soxhlet (containing algae and hexane). |
| |
| • After some time algal oil will be collected from collecting flask. |
| |
| • Algal oil will be further use for biodiesel production. |
| |
| Ultrasonic-assisted extraction [20] |
| |
| • Take 50 gm of dried algae and add 50 ml of ether. |
| |
| • After that provide ultra-sonic wave for about 10 minutes, ( ultrasonic waves are used to create cavitation bubbles in a solvent material) |
| |
| • Ultra-sonic wave will be also work as cell wall rapture of algae. |
| |
| • Filter it with sieves. |
| |
| • After that manually press algae to extract algal oil. |
| |
| • Algal oil will be use to further transesterification process. |
| |
| Lipid analysis |
| |
| Thin layer chromatography |
| |
| 1. Using the template provided, mark the plates with a sharp pencil, |
| |
| 2. Line the chamber with chromatography paper. Prepare 202 ml of solvent system (Hexane:Ether:Acetic acid 60:40:1) in a 500 ml Erlenmeyer flask. Mix and pour ~150 ml into the chamber. Cover and let the chamber saturate while loading the plates. |
| |
| 3. With a 10 μl capillary pipette, spot 1-2 μl of phospholipids standard onto the TLC plate, as shown. Make sure the spot remains smaller than 4 mm in diameter. Move on to the other standards. After the spots have dried, repeat loading each standards until you have loaded approx. 10 μl each. Also, load 10 μl of your lipid extract on one spot, and then the remainder of the extract as a line (i.e. a series of spots). |
| |
| 4. Let dry the spots. Make sure that the loading area is above the solvent. Place the plates in the chamber to develop. |
| |
| 5. Immediately close the cover and let run for approximately 30 min, until the solvent front has reached the upper line. |
| |
| 6. Remove the plate and leave to dry in the rack in the fume hood. Discard the solvent in the waste container provided, remove the chromatography paper and leave in the chamber. Leave the chamberin the fume hood to dry. Now place the plate in the iodine tank in the fume hood. You will see the lipids as yellow spots after about 5 min or so. |
| |
| 7. Mark the edges of the spots with a pencil. Make a tracing on onion skin paper for a record. Crap off lipid fractions as shown, place in weighing paper, fold and roll to grind the clumps. |
| |
| Paper chromatography [21] |
| |
| • Take the Whatmann No.1 chromatography paper of appropriate size |
| |
| • Place it on a rough paper and with the help of pencil and scale draw a line leaving 1.5 cm from the bottom. |
| |
| • Now on the line mark two spots leaving 1.5 cm on either side of the edges. |
| |
| • Now measure the distance between the spots and divide it into four equal parts and mark three more points, in total I have get one points in each paper. |
| |
| • Carefully draw three small circles touching the line, below the line under each circle write the name of the standard. |
| |
| • Sample has loaded in center of the paper. With the help of capillary tube apply standard and the sample give a feather touch and see that the solute do not spread below the line. |
| |
| • Now fold the paper in the form of a cylinder and staple at three different positions with the help of stapler. While stapling it, be careful and check that the two ends of the paper are equal and the spots are present outside the circle and there is a gap between the two edges. |
| |
| Gas chromatography |
| |
| Transesterification [22] |
| |
| • 100 ml Algal oil kept in conical flask. |
| |
| • Add 1ml KOH. (Algal oil will be highly viscous, one of the most common method will be using to reduce oil viscosity in the algae oil is called transesterification. It involves chemical conversion of the oil into its corresponding fatty ester.) |
| |
| • Heat it with 70°C on heating mental and after 2 hour I have collect biodiesel from bioreactor. |
| |
| Results and Discussion |
| |
| Sample collection |
| |
| Algal sample ware collected from different places of bhopal region (Figure 1). |
| |
|
|
Figure 1: Different sample collection. |
|
| |
| Identification of suitable strain: Scandasmus dimorphus algal species was grown on MBM medium and CHU13 medium.images shows below (Figure 2). |
| |
| |
|
|
Figure 2: Culture of different algal sample. |
|
| |
| Algal strain growth was obtained in Hosangabad sample. Select hosangabad sample and grown it 500 ml MBM solution (Figure 3). |
| |
|
|
Figure 3: Media containing algal strain. |
|
| |
| Algae harvesting |
| |
| Micro-screening: Algae harvesting was done after algal culturing by using sieves (250 μ, 500 μ) (Figure 4) |
| |
|
|
Figure 4: Algae harvesting by sieves. |
|
| |
| Centrifugation: In case of low growth rate of desire algal strain I was used centrifuge for algae harvesting (Figure 5). I was take pallets and shaded dry it using lyiphilizer and then make it in powder form. |
| |
|
|
Figure 5: Algal pallets are shown in the bottom. |
|
| |
| Algal oil extraction |
| |
| Ultrasonic-assisted extraction: I was used ultrasonicator for algal oil extraction, upper layer are algal oil shown in the (Figure 6). |
| |
|
|
Figure 6: Ultrasonic-assisted extraction. |
|
| |
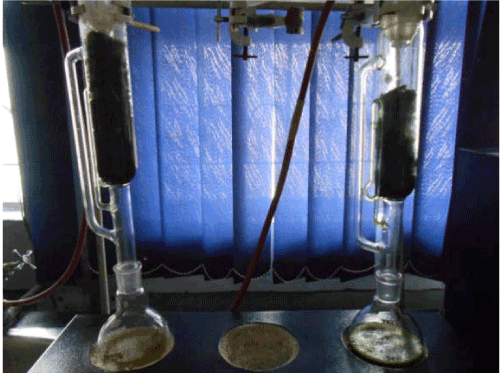
| Soxhlet method for oil extraction: I have used soxhlet apparatus for the extraction for oil from algae (Figure 7). |
| |
|
|
Figure 7: Soxhlet apparatus. |
|
| |
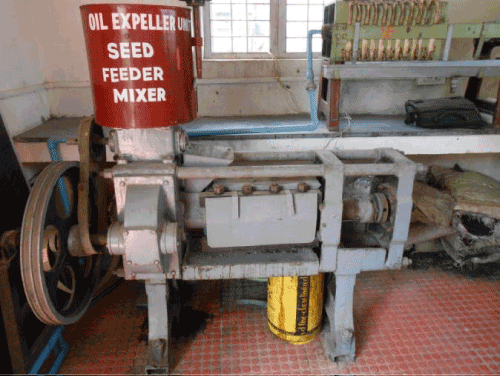
| Expeller Press Method for Oil Extraction: I have used expeller machine for the extraction for oil from algae (Figure 8). |
| |
|
|
Figure 8: Expeller machine. |
|
| |
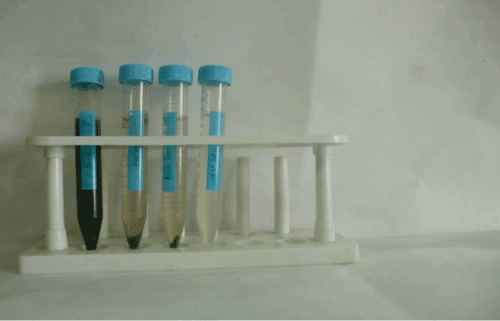
| Oil from different technique (Figure 9, Table 3) |
| |
|
|
Figure 9: Extrected Oil By Different Method. |
|
| |
|
|
Table 3: Oil from Different Techniques. |
|
| |
| Chromatographic technique |
| |
| Thin layer chromatography: RF factor=Distance traveled by solute (cm)/Distance traveled by the solvent (cm) |
| |
| A. RF factor of oil which was extracted by soxhlet method=6/14=0.4285 cm. |
| |
| B. RF factor of oil which was extracted by ultrasonicator method=2.5/13=0.192 cm. |
| |
| C. RF factor of oil which was extracted by expeller method = 3/12= 0.25 cm. |
| |
| Paper Chromatography Technique (Figure 10): RF factor = Distance traveled by solute (cm)/Distance traveled by the solvent (cm) |
| |
|
|
Figure 10: Thin layer chromatographic technique of different oil sample. |
|
| |
| A. RF factor of oil which was extracted by soxhlet method = /= 0.4285 cm |
| |
| B. RF factor of oil which was extracted by ultrasonicator method = /= 0.192 cm |
| |
| C. RF factor of oil which was extracted by expeller method = /= 0. Cm (Table 4) |
| |
|
|
Table 4: RF value of different method. |
|
| |
| Gas chromatography technique |
| |
| Transesterification (Figure 11) |
| |
|
|
Figure 11: Upper layer shows biodiesel and lower shows glycerin. |
|
| |
| Conclusion |
| |
| As demonstrated hear, micro-algal biofuels is technically feasible. It is the only renewable biofuels that can potentially completely relocate liquid fuels derived from gasoline. Financial sides of producing algal biofuels need to recover significantly to be possible. Produce low-cost micro algal biofuels require primarily improvements to micro- algal biology through genetic engineering. Use of the biorefinery idea and advances in photo bioreactors engineering was further lower the cost of fabrication. In view of their much greater yield than raceways, tubular photo bioreactors are likely to be used in producing much of the algal biomass required for making biodiesel. Photo bioreactors provide a prohibited environment that can be modified to the specific stress of highly productive microalgae to attain a consistently good annual give up of algal oil. Algal-biodiesel creation can be done through the use of chemical (acid, base). There is a recent attention in using powerless lipase or immobilized whole cells as “green” alternatives to chemical catalysts to produce biofuels industrially. However, the cost of the final enzymatic product remains a difficulty compared to the cheaper substitute of using chemical catalysis. Using the apparatus of recombinant DNA technology, it is possible to increase the supply of appropriate lipases for biofuels fabrication. Protein engineering and site-directed mutagenesis may be used to alter the enzyme-substrate specificity, stereo specificity, and thermo stability, or to increase their catalytic efficiency, which will benefit biofuels construction, and lower the cost of the generally process. Indeed, a thermo stable and more research efforts should be focused in modifying and cloning more lipases. Lipases can be immobilized to increase their prepared stability, making reuse several times possible, and the alcohol sis process costeffectively reasonable and spirited with straight processes. |
| |
| Acknowledgment |
| |
| The authors thank to the all faculty member of Rajiv Gandhi Proudyogiki Vishwavidiyalya for given support. |
| |
| |
| References |
| |
- Singh J, Gu S (2010) Commercialization potential of microalgae for biofuels production, Renewable and Sustainable Energy Reviews 14: 2596-2610.
- Hammouda O, Gaber A, Abdel-Raouf N (1995) Microalgae and wastewater treatment. Ecotoxicol Environ Saf 31: 205–210.
- Hoffmann JP (1998) Wastewater treatment with suspended and nonsuspended algae. J Phycol 34: 757-763.
- Lopez DE, Goodwin JG, Bruce DA, Lotero E (2005) Transesterification of triacetin with methanol on solid acid and base catalysts. Appl Catal A: Gen 295: 97-105.
- Ma F, Hanna MA (1999) Biodiesel production: a review. Biosour Technol 70: 1–15.
- Freedman B, Pryde EH, Mounts TL (1984) Variables affecting the yields of fatty esters from transesterified vegetable oils. J Am Oil Chem Soc 61: 1638-1643.
- Regan DL, Gartside G (1983) Liquid fuels from micro-algae in Australia, CSIRO publishing: South Melbourne. Victoria, Australia.
- Benemann JR, Oswald WJ (1996) Systems and economic analysis of microalgae ponds for conversion of CO to biomass. US Department of Energy, University of California, Berkeley, USA.
- Park PW, Goins RE (1994) In situ preparation of fatty acid methyl esters for analysis of fatty acid composition in foods. J Food Sci 59: 1262–1266.
- Lepage G, Roy CC (1984) Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J Lipid Res 25:1391-1396.
- Rodríguez-Ruiz J, Belarbi E, Sánchez JLG, Alonso DL (1998) Rapid simultaneous lipid extraction and transesterification for fatty acid analyses. Biotechnol Tech 12: 689-691.
- Doornbosch R, Steenblik R (2007) Round table on Sustainable Development, Biofuels: Is the Cure Worse than the Disease? OECD, Paris.
- Searchinger T, Heimlich R, Houghton RA, Dong F, Elobeid A, et al. (2008) Use of US croplands for biofuels increases greenhouse gases through emissions from land-use change. Science 319: 1238-1240.
- Renewable Fuels Agency (2008) The Gallagher Review of the indirect effects of biofuels production. RFA, London.
- Sylvester-Bradley R (2008) Critique of Searchinger and related papers assessing indirect effects of biofuels on land-use change, ADAS, Boxworth, England.
- Wiggins S, Fioretti E, Keane J, Khwaja Y, McDonald S (2008) Review of the indirect effects of biofuels: Economic benefits and food insecurity, Report to the Renewable Fuels Agency, Overseas Development Institute, London.
- Ivanic M, Martin W (2008) Implications of Higher Global Food Prices for Poverty in Low-Income Countries. The World Bank Development Research Group Trade Team, World Bank, Washington DC.
- Semino S, Joensen L, Winjstra E (2007) Unsustainable Proposal: The Production of Raw Materials for Future Biofuel Processing Plants in Entre Ri´os. Grupo De Reflexio´n Rural.
- Shelef G, Sukenik A, Green M (1984) Microalgae Harvesting and Processing: A Literature Review. A Subcontract Report. Technion Research and Development Foundation Ltd, Haifa, Isarael.
- https://www.oilgae.com/algae/oil/extract/mec/mec.html
- Amsterdam BV (1985) Paper chromatography of the essential oils occurring in the genus Stachys. Journal of Chromatography 333: 288-289 .
- Sree R, Seshu BN, Prasad PSS, Lingaiah N (2009) Transesterification of edible and non-edible oils over basic solid Mg/Zr catalysts. Fuel processing technology 90: 152-157.
|
| |
| |