| Research Article |
Open Access |
|
| G. S. Uthayakumar* and A. Sivasubramanian |
| Electronics and Communication Engineering, St. Joseph’s College of Engineering, Chennai, India |
| *Corresponding authors: |
G.S. Uthayakumar
Electronics and Communication Engineering
St. Joseph’s College of Engineering
Chennai, India
E-mail: g_uthayakumar@hotmail.com |
|
| |
| Received September 03, 2012; Published September 28, 2012 |
| |
| Citation: Uthayakumar GS, Sivasubramanian A (2012) Fiber Optic Transillumination Imaging System and Human Body Relations. 1:376. doi:10.4172/scientificreports.376 |
| |
| Copyright: © 2012 Uthayakumar GS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| A non-invasive technique for the diagnostic and prognosis of gastric problem is proposed. Such technologies would allow for accuracy in results over huge number of patients. In India, there are two types of Medical Treatment Systems (MTS) using various drugs namely, English Medical Treatment Systems (EMTS) and Ayurveda, Unani and Siddha Treatment System (AUSTS). Due to modern food habits, 70% of the population suffers from gastric problem. To find the characteristics and properties of drugs given for the treatment of gastric problem by both type of physicians, the samples are taken for experiment using Fourier transform Infra-red spectrometer. The spectral analysis indicated that the specific functional groups of the drug materials have almost the same chemical characteristics. |
| |
| Keywords |
| |
| Fourier transform infrared spectroscopy; Medical treatment system; Ayurveda; Unani and siddha treatment system; Potassium bromide; Fourier transform |
| |
| Introduction |
| |
| Plants have been used in traditional medicine for several thousand years. In India, medicinal plants as a group comprise approximately 8000 among which 50% accounts for all the higher flowering plant species [1]. The knowledge of medicinal plants has been accumulated in the course of many centuries based on different medicinal systems [1] such as Ayurveda, Unani and Siddha. In a large number of countries, human population depends on medicinal plants for treating various illnesses as well as a source of livelihood. The World Health Organization (WHO) estimated that 80% of population of developing countries relies on traditional medicines, mostly plant drugs, for their primary health care needs. The objective of this research is to identify various chemical groups present in the five important medicinal drugs given for gastric problems by both types of physicians. The number of reported cases of gastric problem [2] is steadily increasing in both industrialized and developing countries. In spite of the fact, significant progress has been achieved in identification of gastric problem at molecular level using FTIR (Fourier Transform Infra Red) spectroscopy and other advanced technologies of bio-optics. Spectroscopic investigations on pharmaceutical samples are of importance in the present. Vibrational spectral studies of many pharmaceutical drugs are extensively studied by many scientists. But so far nobody has made an attempt to study the chemical characteristics [2] of brown sample and green sample. |
| |
| Experimental setup |
| |
| FTIR: In FTIR, the design of the optical pathway produces a pattern called an interferogram. The interferogram is a complex signal, but its wave like pattern contains all the frequencies that make up the infrared spectrum. A mathematical operation known as Fourier Transform (FT) can separate the individual absorption frequencies from the interferogram producing a spectrum virtually identical to that obtained with a dispersive spectrometer. The advantage of an FTIR instrument is that it acquires the interferogram [3] in less than a second. It is thus possible to collect dozens of interferogram of the same sample and accumulate them in the memory of a computer. When a Fourier transform is performed on the sum of the accumulated interferogram, a spectrum with a better SNR can be plotted. An FT-IR instrument is capable of greater speed and sensitivity than the dispersion instrument. There are three basic spectrometer components in an FTIR system: radiation source, interferometer, and detector. The same types of radiation sources are used for both dispersive [3] and Fourier Transform spectrometers. The source is more often water-cooled in FTIR instruments to provide better power and stability. In contrast, a completely different approach is taken in an FTIR spectrometer to differentiate and measure the absorption at component frequencies. The monochromator is replaced by an interferometer, which divides radiant beams, generates an optical path difference between the beams [ 4], then recombines them in order to produce repetitive interference signals measured as a function of optical path difference by a detector. |
| |
| As the name implies, the interferometer produces interference signals, which contain infrared spectral information generated after passing through a sample. The most commonly used interferometer is a Michelson interferometer. It consists of three active components: a moving mirror, a fixed mirror and a beam splitter. The two mirrors are perpendicular to each other. The beam splitter is a semi reflecting device and is often made by depositing a thin film of germanium onto a flat KBr substrate. Radiation from the broadband IR source is collimated and directed into the interferometer, and impinges on the beam splitter. At the beam splitter, half the IR beam is transmitted to the fixed mirror and the remaining half is reflected to the moving mirror. After the divided beams are reflected from the two mirrors, they are recombined at the beam splitter. Due to changes in the relative position of the moving mirror to the fixed mirror [5], an interference pattern is generated. The resulting beam then passes through the sample and is eventually focused on the detector. For an easier explanation, the detector response for a single-frequency component [3] from the IR source is first considered. |
| |
| An infrared (IR) beam from a Michelson interferometer is incident on the crystal interface at angles below critical and undergoes total internal reflection. An evanescent wave is created within a thin layer near the surface and is absorbed by the molecules present. In the interferometer, a moving mirror varies the length of one optical path relative to the other and creates an interferogram that is converted to an absorbance spectrum by a Fourier transform. The two beams are totally in phase with each other; thus, they interfere constructively and lead to a maximum in the detector response. This position of the moving mirror is called the point of Zero Path Difference (ZPD). When the moving mirror travels in either direction by the distance λ/4, the optical path (beam splitter–mirror–beam splitter) is changed by 2(λ/4), or λ/2. The two beams are 180° out of phase [6] with each other, and thus interfere destructively. As the moving mirror travels another λ/4, the optical path difference is now 2 (λ/2), or λ. The two beams are again in phase [7] with each other and result in another constructive interference. |
| |
| Detector: When the mirror is moved at a constant velocity, the intensity of radiation reaching the detector varies in a sinusoidal manner to produce the interferogram output. The interferogram is the record of the interference signal. It is actually a time domain spectrum and records the detector response changes versus time [8] within the mirror scan. If the sample happens to absorb at this frequency, the amplitude of the sinusoidal wave is reduced by an amount proportional to the amount of sample in the beam. Extension of the same process to three component frequencies results in a more complex interferogram, which is the summation of three individual modulated waves. In contrast to this simple and symmetric interferogram, the interferogram produced with a broadband IR source displays extensive interference patterns. It is a complex summation of superimposed sinusoidal waves, each wave corresponding to a single frequency. When this IR beam is directed through the sample, the amplitudes of a set of waves are reduced by absorption if the frequency of this set of waves is the same as one of the characteristic frequencies of the sample. The interferogram contains information over the entire IR region to which the detector is responsive. A mathematical operation known as Fourier transformation converts the interferogram (a time domain spectrum displaying intensity versus time within the mirror scan) to the final IR spectrum, which is the familiar frequency domain spectrum showing intensity versus frequency. The detector signal is sampled at small, precise intervals during the mirror scan. The sampling rate is controlled by an internal, independent reference, a modulated monochromatic beam from a Helium Neon (HeNe) laser [8] focused on a separate detector. |
| |
| Materials and Methods |
| |
| Molecular composition |
| |
| The Brown sample consists of the following composition of traditional plants such as Piper nigrum: 10 mg, Glycyrrhiza glabra: 200 mg, Aloe vera: 5 mg, Embelia ribes: 20 mg, Elettaria cardamom: 20 mg, Myrstica fragrans: 20 mg, Caryophyllus aromatious: 20 mg, Perula foetida: 20 mg, Phyllanthus emblica: 20 mg, Coriandrum sativum: 20 mg, Musa paralisica: 10 mg, Cinnamomum zevlanica: 10 mg, Zingiber efficinate: 10 mg. Orange sample consists of the following composition Ispaghula Husk (Mentago ovata): 85.57%, Methylparaban: 0.2%, Propylparaban: 0.02%. The composition of green sample each 1 ml (20 drops approx.) contains: Pudina Satva: 0.0337 ml (Mentha piperata), Purified Water Q.S, Alcohol IP: 10%, V/V Colors: Tartrazine Yellow Spra, C.I.No.19140: 0.0585 mg, Alizarine Cyanine Green: F.C.No. 61570:0.0553 mg. The molecular formula of one of the composition of Blue sample is 1-[5-(1,3-Benzodioxol-5-yl)-1-oxo-2,4-petadienyl] C 17H19NO3 can be isolated from black pepper (Piper nigrum) and other Piper species. Black pepper contains 6% to 9% piperine by weight. Piperine is tasteless, but its stereoisomer, chavicine, is the active ingredient in black pepper that provides its characteristic taste. Loss of pungency during storage of black pepper is attributed to the slow isomerization of chavicine into piperine. When all these chemical compounds react with the macromolecules such as proteins, fatty acids and glucose, the entire structure is changed and sometimes it gives excellent results for gastric problem. |
| |
| Potassium bromide pellet |
| |
| Potassium bromide pellets are used to obtain the Fourier Transform Infrared spectra for solids such as orange, yellow, green, blue and brown samples. KBr is an inert, infrared transparent material, and acts as a support for the sample. There are two steps to follow for preparing successful KBr pellets. First, the sample and the KBr must be ground to reduce the particle size to less than 2 microns in diameter. Grinding is traditionally performed with an agate mortar and pestle, but a WIG-LBUG may also be used. A gram of KBr should be placed in the mortar. It should be ground until crystallites can no longer be seen and it becomes somewhat “pasty” and sticks to the sides of the mortar. The KBr and the sample should be ground separately to avoid possible chemical interactions, the heat and pressure generated in the mortar may cause the KBr to react with the sample. |
| |
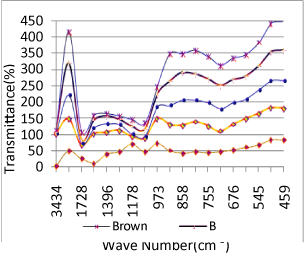
| The spectrum obtained may be that of the product of this reaction rather than the original sample [1-5]. After grinding the sample and KBr, the sample is diluted to about 1% in the ground KBr. About 1 to 10 mg of sample can be used. The amounts of material to use can be “eyeballed” with some success, but actually weighing but the amounts will give more reproducible results. It is important that the sample should be well dispersed in the KBr. Mixing the sample and KBr in the mortar for a minute using a spatula is usually sufficient to ensure good dispersion. The sample/KBr mixture is then placed in a dye or press, and is squeezed to produce a transparent pellet. Several tons of pressure may be necessary to obtain a transparent pellet. If the pellet is inside a dye, place the pellet/dye combination directly in the FTIR beam. KBr is a hygroscopic material, which means it will absorb water directly from the atmosphere. It is critical that the KBr used in making pellets should be kept warm and dry, preferably at >100°C. Cloudy regions in a pellet indicate that the pellet has absorbed water [9], which will give bands around 3900 and 1630 cm-1.The Table 1 shows the frequency and wavelength of all the five samples taken for experiment. The similarities of some chemical compounds are highlighted in blue color. From Table 2, the bands exhibited in the region around 3400 to 3390 cm-1 can be assigned to N-H stretching for all the five samples and also vibrational frequencies exhibited from 1600 to 1655 cm-1 are considered to be due to C-H stretching of the compound. |
| |
|
|
Table 1: Wave number in frequency and wavelength for Orange sample. |
|
| |
|
|
Table 2: Wave number in frequency and wavelength for Blue sample. |
|
| |
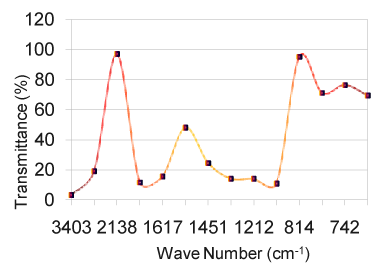
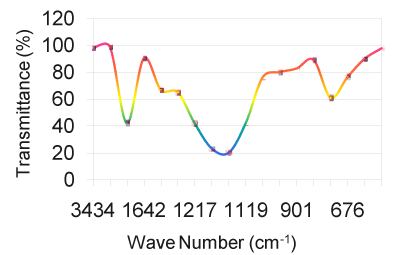
| The relative intensity for both the regions is strong. From the region 1000 to 1200 cm-1, the vibrational frequencies are assigned to C-O-C stretching due to polysaccharide functional groups. N-H wagging is appeared for all the samples at 750 to 775 cm-1. The N-H stretching absorption is less sensitive to hydrogen bonding than O-H absorptions. In the gas phase and in dilute CCl3 solution free N-H absorption is observed in the region from 3400 to 3500 cm-1 (Table 1). C-N stretching absorptions are found at 1200 to 1350 cm-1 for aromatic amines and at 1000 to 1200 cm-1 for aliphatic amines. The IR spectrum of a compound is the superposition of the absorption bands of specific functional groups. As such, the IR spectrum can be used as finger print for identification of unknown in comparison with previously recorded reference spectra. By observing the position, shape and relative intensities of the vibrational bands in FTIR spectra of the drugs, a satisfactory vibrational band assignment has been made. The FTIR spectra of the sample orange drug are presented in figure 6. The vibrational band assignment of the drugs is summarized in table 1 and is discussed as follows. The bands exhibited in the region around 3000 cm-1 can be immediately assigned [10] to be due to aromatic C-H stretching (Table 2). In this view, the vibrational frequencies exhibited at 3434 cm-1 in the FTIR spectrum are considered to be due to C-H stretching vibrations of the compound. The C-C ring stretching vibrations occur in the region from 1642 to 1579 cm-1 in FTIR spectra. |
| |
|
|
| |
| Calculation |
| |
| It is possible to calculate the value of a stretching vibrational frequency of a bond by use of Hook’s law which may be represented as: |
| |
 |
| |
| Where, |
| |
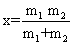 |
| |
| It is the reduced mass, m1 and m2 are the masses of atom in a particular band and k is the force constant. It is regarded as a measure of stiffness of the bond. For a single bond, it is approximately 5×105 dynes/ cm. Its value becomes double and triple for a double and a triple bond, respectively. If we consider a diatomic molecule having resultant dipole moment, the vibratory motion of the nuclei of such a molecule may be similar to that of a linear harmonic oscillator. If the bond between two nuclei of diatomic molecule is distorted from equilibrium length L0 to a new length L, the restoring forces on each atoms of diatomic molecule (Equations. (1) and (2)) will be given by |
| |
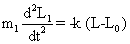 |
| |
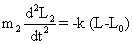 |
| |
| where L1 and L2 are positions of atoms 1 and 2 relative to the center of gravity of the molecule. Hence, |
| |
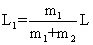 |
| |
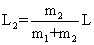 |
| |
| From Equations (1) and (3) we get |
| |
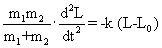 |
| |
| Since L0 is constant, hence |
| |
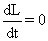 |
| |
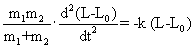 |
| |
| From equations (5) and (6), we get |
| |
| Substitute |
| |
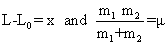 in Eq. (6) in Eq. (6) |
| |
 |
| |
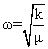 |
| |
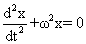 |
| |
| Results and Discussions |
| |
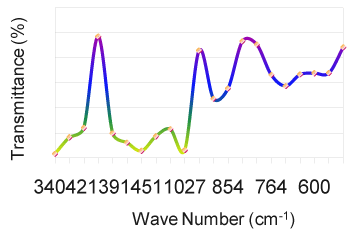
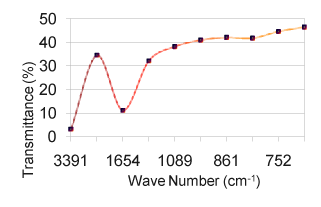
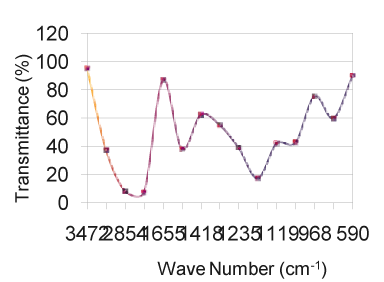
| As solids or liquids, primary aliphatic amines absorb in the region from 3434 cm-1 to 3450 cm-1 (Figure 1) and exhibit a broad band of medium intensity. In dilute solution in non-polar solvents, two bands are observed for primary amines due to N-H asymmetric and symmetric vibration in the range from 3550 cm-1 to 3250 cm-1 (Table 5). The relative intensity of the band due to the hydroxyl stretching decreases with increase in concentration with additional broader bands appearing at lower frequencies from 3580 cm-1 to 3200 cm-1. In aminobenzoesaesure, the hydroxyl stretching [11] occurs at 3434 cm-1 in FTIR spectra. From the above band assignments, the sample under experiment shows a very strong band at 3434 cm-1 in FTIR spectrum due to N-H and O-H stretching (Figure 2). |
| |
|
|
| |
| The carbonyl groups exhibits a strong absorption band due to C=O stretching vibration at 1728 cm-1. In fluorouracil, the bands observed at 1642 cm-1 in FTIR [12] is assigned to C=O asymmetry stretching vibration. A strong band at 1728 cm-1 is assigned for C=O carbonyl [ 13] stretching of nalidixic acid. The band at 1642 cm-1, which is close to the literature range due to characteristics [14] are assigned for C=O stretching in benzocaine. Keeping this in mind, the sharp band present in the expected region at 1728 cm-1 in the FTIR spectrum is allotted to be due to C=O stretching vibration (Figure 3). C–N stretching absorption of primary aliphatic amines is weak and occurs in the region from 1119 cm -1 to 973 cm-1. Secondary aliphatic amines have bands of medium intensity at 1178 cm-1 to 1140 cm-1 (Table 3). The band in the region 1217 cm-1 in FTIR have assigned to C–N symmetry stretching [12] of the compound fluorouracil. In this analogy, the bands at 1217 cm-1 in FTIR spectra of the drug sample is assigned due to C–N vibrations. The bands due to C–O stretching vibrations are strong and occur in the region from 1217 cm-1 to 1119 cm-1(Figure 4). |
| |
|
|
| |
|
|
Table 3: Relationship between wave number and frequency for Yellow sample. |
|
| |
|
|
| |
| In aminobenzoesaeure, the strong band which occurs near 1119 cm-1 in the FTIR spectra [11] is assigned to C–O stretching vibration. In benzocaine, the very strong and sharp peak at 1217 cm-1 has been assigned to the C–O stretching. Taking the above band assignments, 1119 cm-1 and 973 cm-1 (Table 5) in FTIR spectrum of the sample under experiment are assigned due to C–O vibration. A number of C–H in plane deformation bands occur in the region from 973 cm-1 to 901 cm-1, the bands being sharp but weak to medium intensity. However, these bands are not normally important for interpretation purpose although they can be used. The aromatic C–H out of plane deformation bands occur below 700 cm-1 (Table 4). The bending vibration is generally found at lower wave numbers. The frequencies observed at 775 cm-1, 705 cm-1, 676 cm-1, 592 to 491 cm-1 are assigned to be due to O=C–C , O=C–N , C=C–N and C=C–C bending(Figure 6) of the pyrimidine ring in the FTIR spectra of Xanthine and C–N–C bending vibrations are assigned at 498 cm-1 and 428 cm-1 [15]. Using the above analogy, the bands at 901 cm-1 to 775 cm-1 is due to C–H in plane deformation. The bands at 676 cm-1 is due to C–H out of plane deformation/C–C=O deformation (Figure 5). |
| |
| |
|
|
Table 4: Relationship between wave number and frequency for Brown sample. |
|
| |
|
|
Table 5: Relationship between wave number and frequency for Green sample. |
|
| |
|
|
| |
|
|
Figure 6: For all the samples. |
|
| |
| Conclusion |
| |
| Orange color sample is an antibiotic for gastric trouble given by physician for english medical treatment system and the yellow color sample is given for liver function problem. The remaining three drugs such as green sample, blue sample and brown sample are given for gastric problem by Ayurveda, Siddha and Unani treatment systems. An attempt has been made in this work to study the vibrations of the functional derivatives of all the five samples. By observing the position, shape and relative intensities of the vibration bands in FTIR spectra of all the drugs, a satisfactory vibration band assignment has been made. FTIR analysis was conducted to verify the occurrence of chemical bonds between the English medical drug and Ayurveda, Siddha and Unani medical treatment drugs. The spectral analysis indicated that the specific functional groups of the both types of drugs have almost the same chemical characteristics. The research reviews suggest that molecular interaction always could alter the chemical structure of the drug. As per the results of both types of medical treatment systems existing in India, it is concluded that both types of drugs have the same chemical bonds and characteristics but different reactions at molecular level in the human body. |
| |
| Acknowledgement |
| |
| I would like to acknowledge Mr. Shankar, Technical Assistant, Sophisticated Analytical Instrument Facilities (SAIFs) at IIT, Chennai, for providing the FTIR equipment for the complete analysis of both types of samples. |
| |
| |
| References |
| |
- Bright A, Renuga Devi TS, Gunasekaran S (2010) Spectroscopical vibrational band assignment and qualitative analysis of Biomedical compounds with cardiovascular activity. Int J Chem Tech Res 2: 379-388.
- Chamundeeswari M, Sobhana SS, Jacob JP, Kumar MG, Devi MP, et al. (2010) Preparation, characterization and evaluation of a biopolymeric gold nanocomposite with antimicrobial activity. Biotechnol Appl Biochem 55: 29-35.
- Miller JN, Miller JC (2000) Statistics and Chemometrics for Analytical Chemistry. (4thedn), Prentice Hall.
- Monograph Committee, Malaysian Herbal Monograph, National Pharmaceutical Control Bureau (2001). Ministry of Health Malaysia, Vol. 2.
- Caubet C, Bousset L, Clemmensen O, Sourigues Y, Bygum A, et al. (2010) A new amyloidosis caused by fibrillar aggregates of mutated corneodesmosin. FASEB J 24: 3416-3426.
- Chew OS, Hamdan MR, Zhari I, Ahmad MN (2003) Nineteenth Annual Seminar & Workshop of the Malaysia Natural Products Society. Faculty of Science,University of Malaya, Malaysia.
- David-Vaudey E, Burghardt A, Keshari K, Brouchet A, Ries M, et al. (2005) Fourier Transform Infrared Imaging of focal lesions in human osteoarthritic cartilage. Eur Cell Mater 10: 51-60.
- Irishina N, Moscoso M, Dorn O (2009) Microwave imaging for early breast cancer detection using a shape-based strategy. IEEE Trans Biomed Eng 56: 1143-1153.
- Chen R, Huang C, Ke Q, He C, Wang H, et al. (2010) Preparation and characterization of coaxial electrospun thermoplastic polyurethane/collagen compound nanofibers for tissue engineering applications. Colloids Surf B Biointerfaces 79: 315-325.
- Gunasekaran.S, Abitha.P (2005) Fourier transform infrared and FT-Raman spectra and normal coordinate analysis of aminobenzoesaesure. Indian Journal of Applied Physics 43: 329.
- Gunasekaran.S, Ponnambalam.U, Muthu.S, Kumaresan.S. (2009) FTIR, FTIR Raman Spectra and molecular structural confirmation of isoniazid. Indian Journal of Applied Physics 47: 12-18.
- Renuga Devi TS (2007) Spectroscopic analysis of lipid disorders and study in the quality and efficacy of status and fibrates, Ph. D. Thesis, University of Madras.
- Gunasekaran S, Sankari G, Ponnusamy S (2005) Vibrational spectral investigation on xanthine and its derivatives--theophylline, caffeine and theobromine. Spectrochim Acta A Mol Biomol Spectrosc 61: 117-127.
- Dhanikula AB, Panchagnula R (2008) Fluorescence anisotropy, FT-IR spectroscopy and 31-P NMR studies on the interaction of paclitaxel with lipid bilayers. Lipids 43: 569-579.
- Silerstein RM, Clayton OG, Morril T (1981) Spectroscopic Identification of Organic Compounds 4E: John Wiley, New York.
|
| |
| |