| Research Article |
Open Access |
|
| Ahmad Z*, Senthil Kumar A and Krishnasamy U |
| School of Chemical Engineering, Universiti Sains Malaysia, Engineering Campus, Seri Ampangan, 14300 Nibong Tebal, Seberang Perai Selatan, Pulau Pinang, Malaysia |
| *Corresponding authors: |
Zainal Ahmad
School of Chemical Engineering
Universiti Sains Malaysia, Engineering Campus
Seri Ampangan, 14300 Nibong Tebal
Seberang Perai Selatan
Pulau Pinang, Malaysia
Tel: 006-012-7851063
Fax: 006-04-5941013
E-mail: chzahmad@eng.usm.my |
|
| |
| Received September 03, 2012; Published October 18, 2012 |
| |
| Citation: Ahmad Z, Senthil Kumar A, Krishnasamy U (2012) Role of Mixing and Temperature on Molecular Weight Distribution of Polycaprolactone Synthesized from ε-Caprolactone. 1:379. doi:10.4172/scientificreports.379 |
| |
| Copyright: © 2012 Ahmad Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| The effect of temperature and mixing on Ring-Opening Polymerizations (ROP) of ε-caprolactone was studied by using lipase enzyme Novozym 435 (immobilized form of lipase B from Candida antarctica) as biocatalyst. The polymerization of ε-caprolactone was carried out at various impeller speeds of 250, 500, 750 and 1000 rpm where in the temperature of the reactor were maintained at 60°C, 70°C and 80°C. The maximum molecular weight out of all the experiments carried out is 310000 KDa which was obtained at a temperature of 70°C and 3 hours for an impeller speed of 500 rpm. The results also indicate that increasing the speed reduces the molecular weight distribution. The conclusion is that 500 rpm is found to be the most appropriate speed for the impeller that needs to be maintained for the enzymatic catalyzed polymerization process because when the impeller speed is increased to 1000 rpm the trend seems to vary which might be due to degradation occurring or in other words an increase in impeller speed could lead to killing of the enzymes. |
| |
| Keywords |
| |
| Enzymatic Polymerization; Ring Opening Polymerization (ROP); Polycaprolactone Synthesis; Mixing; Temperature; Molecular Weight Distribution. |
| |
| Introduction |
| |
| Recently a large amount of focus has been in the field of enzymatic catalyzed polymerization, the reason being that enzymes as catalyst have been more eco-friendly when compared to that of conventional polymerization where in the inorganic catalysts which are of toxic and precious materials. Even though enzymatic polymerizations seem to be a very good alternative process, its high cost has taken it to the back seat as the cost of production and purification has taken the toll. Hence a polymerization process to produce a polymer like polyester with commercial enzyme as catalyst at a cheaper price would be our objective and to achieve this, the first step would be to optimize the parameters involved in the process. The commercial lipase enzyme (Novozym435) is used as a catalyst in converting ε-caprolactone to polycaprolactone using Ring Opening Polymerisation (ROP) in an enzymatic catalyzed polymerization process where optimization of the reactor parameters namely temperature, impeller speed are taken into consideration in the present study. |
| |
| Poly (e-caprolactone) is an important environment-friendly polymer. The ROP of 5-keto-e-caprolactone has attracted attention for several reasons: the monomer can be synthesized in high yield, it has sufficient ring strain to enable homopolymerization and either the monomer or polymer can be further derivatized. The efficient monomer synthesis was a very important factor, Poly e-caprolactone (PCL) is semi-crystalline in nature and has several important properties such as biodegradability, biocompatibility, good mechanical properties and its molecular weight can easily be controlled to ensure optimum performance. The aliphatic polyester PCL and its copolymers are of great interest for applications in biological and biomedical areas. |
| |
| Hence this novel approach has been dealt by Gross & Kumar [1] and also of Madras & Sivalingam [2]. Their work dealt with the enzymatic catalyzed polymerization process of converting ε-caprolactone to polycaprolactone using ring opening polymerisation where their main objective was the optimization of the parameters namely solvent, temperature, speed using the small scale experiments. Hence moving ahead with the large scale production of polycaprolactone remains a target while optimizing the parameters temperature, impeller speed remains a challenge. |
| |
| Whenever a process needs to be scaled up the optimization has always been a challenge as the composition, impeller speed, temperature that were considered for the small scale laboratory work of the same process would not be suitable for the large scale up. The history of ROP is vast and old, to name a few, studies of enzyme catalyzed lactones ROP in organic media have been conducted for the polymerizations of ε-caprolactone (ε-CL) [3-5]. Enzyme-catalyzed polymerizations of macrolactones have already resulted in improved propagation kinetics and/or molecular weights when compared to chemical preparative routes. The enzyme-catalyzed polymerizations of ω-undecanolide (UDL), ω-dodecanolide (DDL), and ω-pentadecanolide (PDL) (12-, 13-, and 16-membered lactones) were first investigated by Kobayashi et al. [6-8]. |
| |
| Even though many researchers have worked in this field, still the scale-up in larger level by optimizing the parameters and to have a control over the process is put forward as a challenge for the next stage of research for efficient production and for the enhanced product performance. |
| |
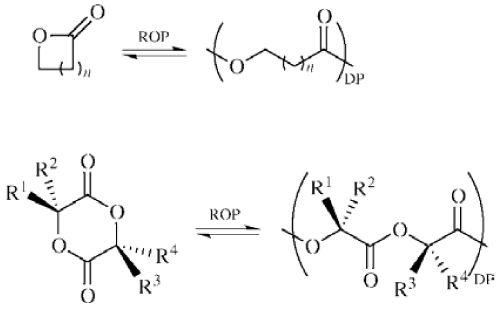
| In particular enzymatic catalyzed polymerization is a fast progressing field, and ROP of ε-caprolactone to produce polycaprolactone with commercial lipase enzyme as catalyst is gaining momentum. ROP is a widely studied polymerization process especially in a stage where conventional polymerization process is under severe scrutiny for its inability to overcome environmental aspects. Polymers prepared by the ROP process are used for a wide range of applications. In practice it is very important to synthesize polymers with a targeted molar mass. It is well known, that the final molar mass of a polymer prepared using the ROP process is defined by the monomer/initiator molar ratio [9-10]. In addition, other factors have also been reported to influence the ROP polymerization process. These include type of polymerization techniques (melt, bulk or solvent) [11], initiator [12], type and concentration of catalyst [13-14], temperature [15], monomer concentration [16], stirring speed [17] and impurities (water and hydroxyl containing substances) [18] (Figure 1). |
| |
|
|
Figure 1: Ring opening polymerization of unsubstituted lactones, lactides, and glycolide. n= 1: β-propionolactone (β-PL); n = 2: γ -butyrolactone (γ -BL); n = 3: δ-valerolactone (δ-VL); n = 4: ε-caprolactone (ε-CL); DP = degree of polymerization. R1 = R2 = R3 = R4 = H: glycolide; R1 = R4 = CH3 and R2 = R3 = H: L-lactide (L-LA); R1 = R4 = H and R2 = R3 = CH3: D-lactide (D-LA); R1 = R3 = CH3 and R2 = R4 = H: meso-lactide (meso-LA); D-LA/L-LA = 50/50: racemic-lactide (D,L-LA). |
|
| |
| In order to optimize the conditions of synthesis, there is a necessity to develop a model that acts as the solution for the optimization problems related to the performance of the reactor. Since the present work deals with polymers, the quality of polymer is a very important factor to be taken into consideration. There are several parameters in the process that affect the polymer quality, namely the temperature, time, mixing parameter/elements and composition. The quality of the polymer is determined by its molecular weight or in other words polymer quality is directly proportional to the molecular weight [19]. As the process is nonlinear, modelling is needed in order to capture the nonlinearity of the system. Similar to that of NN that captures the nonlinearity of the process by having the sigmoid or tansig function in the equation, linearization needs to be carried out in order to reduce the nonlinearity of the system. Modelling actually reduces the number of equation as it is a black box approach. Hence in order to achieve this target initially there is a need for optimization of parameters. |
| |
| The details about the challenges faced by each of the above mentioned parameters are discussed in detail below. |
| |
| Parameters of Interest |
| |
| There have been several attempts [1] that have been made to enhance the understanding of ring-opening polymerization by studying various parameters such as enzyme loading, water content, reaction media, and operating temperature. In this particular work the parameters that have been considered are temperature, time and impeller speed. |
| |
| Temperature |
| |
| The selection of the temperature range was a tedious task as the previous references could be merely used. Hence the range that was selected was 50 to 100°C. This lead to the optimization of the temperature to be 70°C at which the maximum molecular weight was obtained [20]. |
| |
| Time |
| |
| Time is one of the most important parameter in this case study. Due to the lack of any exact reference matching the scale up of the enzymatic catalyzed polymerization process and since the results from the flask level experiments suggests that carrying out the experiment for a longer period of time would only lead to the decrease of the molecular weight as the trend obtained out of the previous experiments showed that the molecular weight increased only to a certain limit followed by its decrease [20]. Hence going by the flask level results which indicated that the maximum molecular weight was obtained at 4hrs and then the molecular weight decreased, this resulted in optimizing the number of hours the bioreactor needs to be run. |
| |
| Impeller speed |
| |
| Whenever a study involving enzymes is carried out, the impeller speed would have a serious effect on the behaviour of the process. The abrupt increase in the speed would affect the enzymatic process and running at a very low speed would affect the objective of obtaining biopolymer of desired quality indicated by its molecular weight. Hence in the present work experiments were carried out with different speeds for different temperatures and as a result the exact speed that would produce the desired output was optimized. |
| |
| Case study: Reactor Control Unit |
| |
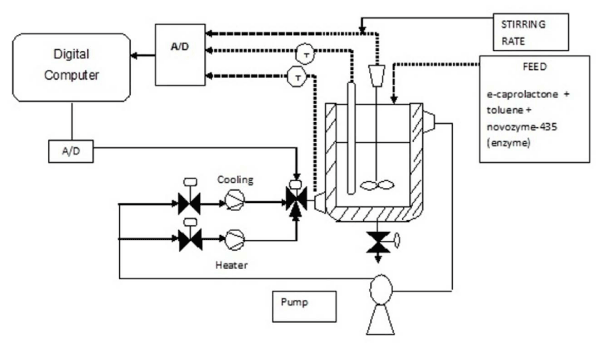
| The apparatus that is on a dry level floor had its 3 phase plug connected to a laboratory 3 phase supply. The power cables were connected from the chiller unit to the power supply (H) located at the side of the control panel. The tank covers were removed and chiller (L), cold water tank (F) and hot water tank (B) were filled with water. Water inlet valve (M) and water outlet valve (O) were opened. The valve to control the water flowrate from the chiller and back to the chiller was regulated. The hose of the water inlet (J) and outlet (K) to the jacketed glass reactor was connected. The water pump at the control panel (G) was turned on. The chiller unit (L) was switched on and temperature was set to the desired value. The heater was turned on and temperature was set to the desired value. The details apparatus is shown in Figure 2. |
| |
|
|
Figure 2: Schematic diagram of batch bio-reactor. |
|
| |
| The solvent to monomer to catalyst ratio taken up for the experiment is 20:10:1. The chemicals used were Toluene, ε-caprolactone, Novozym 435 and the ratio of solvent to monomer to catalyst 2:1:10 (v/v/wt). The effective volume of the reactor is 2 litres, which is the total volume for solvent and monomer. The chemicals and enzyme were fed into the reactor. The reactor was started and the reactor temperature was allowed to rise to the desired value. The stirrer was started and set to the desired speed. As the mixture starts to react and the sample were removed according to the time interval. |
| |
| Zeta-sizer analyser was used for the molecular weight analysis for this research which was performed using Zeta-sizer 1000 HS, Malvern Instruments Ltd., Worcestershire, United Kingdom (UK). The measurement of molecular weight is explained in five different stages. |
| |
| |
| 1. Manual measurement: First the configuring of manual measurement is carried out. Then selection of molecular weight measurement is done. This is followed by entering of sample name, selection of appropriate material and solvent and choosing of standard. |
| |
| 2. Performing of measurement: The reference material set consists of scattering standard and three concentrations of polycaprolactone sample. |
| |
| 3. Measurement: The measurement is started by measuring dark count right which is the count right with no laser. This is followed by measurement of scattering standard which is toluene. These are for the calibration of molecular weight measurement. |
| |
| 4. Next is the analysis of first sample with a specific concentration. The more accurate the concentration is known for molecular weight measurement, the more accurate the results are likely to be. |
| |
| 5. Viewing the results: The record view shows the results of molecular weight measurement. The report shows the trend plot and also the second varial coefficient. |
| |
| Results and Discussion |
| |
| Temperature effects |
| |
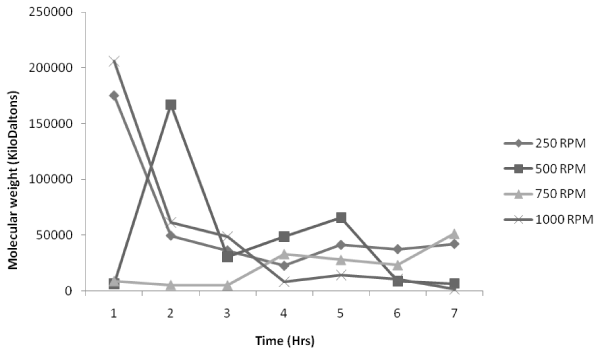
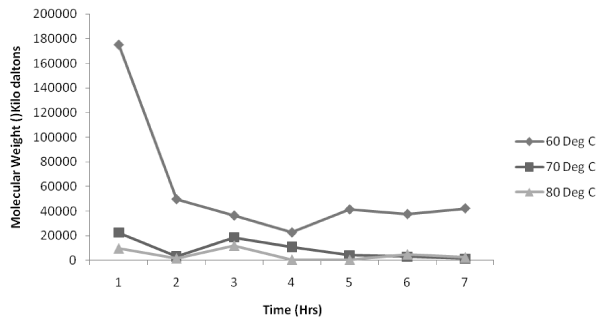
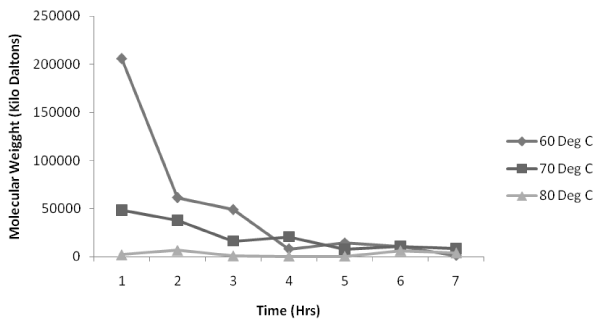
| Figure 3, 4, 5 shows the variation of molecular weight with reaction time at various temperatures (60°C, 70°C and 80°C respectively) with a volume ratio of 1:2 of ε-caprolactone /toluene being taken. Figure 3 indicates that for an impeller speed of 250 rpm and 1000 rpm, the polymerization rates are initially very high at the beginning of the reaction but as the time proceeds the values of molecular weight is found to decrease. But for 500 rpm the polymerization is found to be highest at the 2nd hr of the reaction which is 150000 KDa to be followed by a steady decrease. In case of 750 rpm impeller speed the polymerization is very much unique as the molecular weight achieved is highest at 4th hr which is similar to our experimental study carried out in the flask level [20]. But the inference is that the value of molecular weight is far lesser than other impeller speed. |
| |
|
|
Figure 3: Effect of impeller speed on molecular weight distribution at 60°C. |
|
| |
|
|
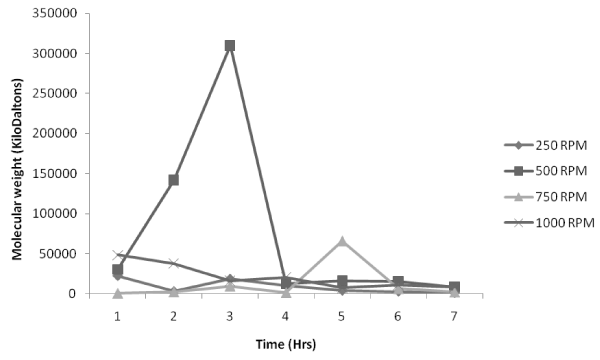
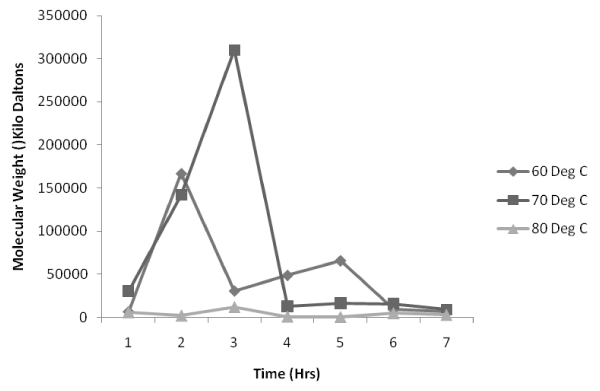
Figure 4: Effect of impeller speed on molecular weight distribution at 70°C. |
|
| |
|
|
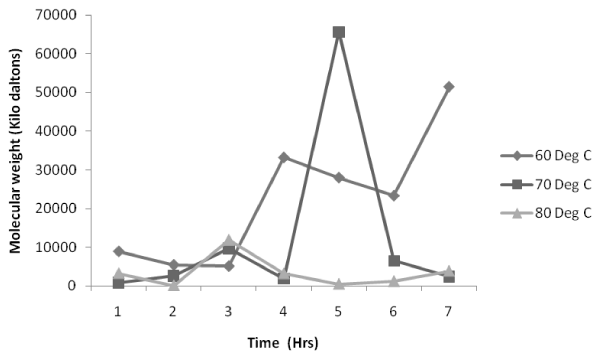
Figure 5: Effect of impeller speed on molecular weight distribution at 80°C. |
|
| |
| Figure 4, which is the variation of molecular weight with reaction time at a temperature of 70°C, shows that 500 rpm impeller speed is most suitable for the process as the value of molecular weight is maximum as high as 310000 KDa compared to that of the molecular weights obtained for 250 rpm, 750 rpm and 1000 rpm. Looking at the trend all the impeller speeds indicate the same trend of maintaining a lower value of molecular weight at the initial reaction time up till 3hrs after which there is an increase to be followed by a decrease in the molecular weight at the completion of the reaction nearing 7th hr. |
| |
| Figure 5 indicates that experiments conducted at various impeller speed have a uniform trend where in the molecular weight is found to be maximum at the reaction time of 3rd hr. However, at the begining of the reaction the molecular weight is higher after which there is a sudden decrease to reach the maximum at the 3rd hr. This is followed by the gradual decrease in molecular weight uptil 6th hr. At the 6th hr there is a sudden increase in molecular weight and to be followed by a decrease at the 7th hr of reaction time. |
| |
| Mixing effect |
| |
| As the previous research in the field of enzymatic catalyzed polymerization have been focusing on the small scale synthesis of polycaprolactone they restricted their objective of using different organic media, subjecting the reacting mixture to different temperature, enzyme effect, dilution effect, not much of interest has been shown in the mixing. But when a large scale production of polycaprolactone is required, an important factor that comes into limelight is the mixing. The reason is that in the small scale where magnetic stirrer was used [ 20] the control over the speed is impossible as the provision for control of speed is not available, but when the bioreactor is used, the role of impeller speed has to be taken into consideration and as a result varying the impeller speed from 250 rpm to 1000 rpm provides an interesting analysis which is explained in details. These results have given a new perspective of the enzymatic catalyzed polymerization field. The conclusions are alarming and as a result will intrigate the researchers to shift their focus to this new parameter or rather a new phenomena. |
| |
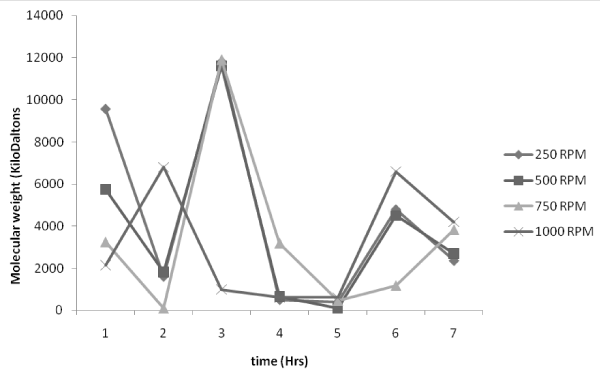
| Figure 6,7,8,9 indicates the variation of molecular weight with reaction time at various impeller speed (250,500, 750, 1000 rpm respectively) with a volume ratio of 1:2 of ε-caprolactone /toluene being taken at a constant temperature that have been considered for the previous set of temperatures namely 60°C, 70°C, 80°C. Figure 6, shows the effect on molecular weight at a particular impeller speed of 250 rpm, the trend followed is the same which indicates that as the reaction starts the process of polymerization at all the temperatures considered namely 60°C, 70°C, 80°C is found to be maximum initially at the 1st hr as the time proceeds the molecular weight is found to decrease steadily. |
| |
|
|
Figure 6: Effect of temperature on molecular weight distribution at 250 rpm impeller speed. |
|
| |
|
|
Figure 7: Effect of temperature on molecular weight distribution at 500 rpm impeller speed. |
|
| |
|
|
Figure 8: Effect of temperature on molecular weight distribution at 750 rpm impeller speed. |
|
| |
|
|
Figure 9: Effect of temperature on molecular weight distribution at 1000 rpm impeller speed. |
|
| |
| In Figure 7 as well the trend is very much uniform where in the plot indicates that maximum molecular weight is achieved at the 3 hrs after maintaining a lower values at the begining of the reaction. Later after the 3 hrs there is a gradual steady decrease in the molecular weight and finally attaining the least value at the end of 7 hours. |
| |
| Figure 8 indicated that there is a very low value of molecular weight polymer produced at a particular impeller speed of 750 rpm for all the three temperature considered namely 60°C, 70°C, and 80°C. |
| |
| Figure 9 indicates the variation of molecular weight for a particular impeller speed of 1000 rpm for various temperatures considered. For all the temperatures considered the maximum value of molecular weight is found to be at the initial reaction time of 1 hr. This was followed by a steady decrease. For 80°C the value of molecular weight throughout the process is found to be very minimal. |
| |
| Conclusion |
| |
| In this paper the effects of temperature and mixing on Ring- Opening Polymerizations (ROP) of ε-caprolactone was studied by using lipase enzyme Novozym 435 (immobilized form of lipase B from Candida antarctica) as biocatalyst where in for an increase in reaction time the conversion increases steadily and after a gradual increase there is a decrease which is found uniform for all the temperature showing a uniform trend. The maximum molecular weight out of all the experiments carried out is 310000 KDa which was obtained at a temperature of 70°C and 3 hours for an impeller speed of 500 rpm. The results also indicate that increasing the speed reduces the molecular weight distribution. But the results also have provided a unique trend where in the molecular weight seems to shoot up to a very high value in the first 1 hour and slowly follows a trend where in the molecular weight seems to achieve a lower value. Hence the overall conclusion is that even though the effect of speed is not highly significant on the molecular weight distribution, the results have been different from that of the results obtained by carrying out in small scale level. The conclusion is that 500 rpm is found to be the most appropriate speed for the impeller that needs to be maintained for the enzymatic catalyzed polymerization process. The reason behind this is that when the impeller speed is increased to 1000 rpm the trend seems to vary which might be due to degradation occurring or in other words an increase of the impeller speed could lead to killing of the enzymes. |
| |
| |
| References |
| |
- Kumar A, GrossRA (2000) Candida antartica lipase B catalyzed polycaprolactone synthesis: effects of organic media and temperature. Biomacromolecules 1: 133-138.
- Sivalingam G, Madras G (2004) Modeling of Lipase Catalyzed Ring-Opening Polymerization of e-Caprolactone. Biomacromolecules 5: 603-609.
- Uyama H, Kobayashi S (1993) Enzymatic Ring-Opening Polymerization of Lactones Catalyzed by Lipase. Chem Lett 22: 1149-1150.
- Knani D, Gutman AL, Kohn DH (1993) Enzymatic polyesterification in organic media. Enzyme-catalyzed synthesis of linear polyesters. I. Condensation polymerization of linear hydroxyesters. II. Ring-opening polymerization of ?-caprolactone. J. Polym Sci Part A: Pol Chem 31: 1221-1232.
- MacDonald RT, Pulapura Sk, Svirkin YY, Gross RA, Kaplan DL, et al. (1995) Enzyme-Catalyzed .epsilon.-Caprolactone Ring-Opening Polymerization. Macromolecules 28: 73-78.
- Uyama H, Takeya K, Kobayashi S (1995) Enzymatic Ring-Opening Polymerization of Lactones to Polyesters by Lipase Catalyst: Unusually High Reactivity of Macrolides. Chem Soc Jpn 68: 56-61.
- Uyama H, Takeya K, Hoshi N, Kobayashi S (1995) Lipase-Catalyzed Ring-Opening Polymerization of 12-Dodecanolide. Macromolecules 28: 7046-7050.
- Uyama H, Kikuchi H, Takeya K, Kobayashi S (1996) Lipase-Catalyzed ring-opening polymerization and copolymerization of 15-pentadecanolide. Acta Polym 47: 357-360.
- Stevens MP (1998) Polymer Chemistry an Introduction. (3rdedn) Oxford University Press, New York, USA.
- Stridsberg K, Ryner M, Albertsson AC (2000) Dihydroxy-Terminated Poly(L-lactide) Obtained by Controlled Ring-Opening Polymerization: Investigation Of Polymerization Mechanism. Macromolecules 33: 2862-2869.
- Veld PJA, Velner EM, Witte van de P, Hamhuis J, Dijkstra P, et al. (1997) Melt block copolymerization of e-caprolactone and L-lactide. J Polym Sci Part A Pol Chem 35: 219-226.
- Pantiru M, Iojoiu C, Hamaide T, Delolme F (2004) Influence of the chemical structure of transfer agents in coordinated anionic ring-opening polymerization: application to one-step functional oligomerization of e-caprolactone. Polym Int 53: 506-514.
- Biela T, Kowalski A, Libiszowski J, Duda A, Penczek S (2006) Progress in Polymerization of Cyclic Esters: Mechanism and Synthetic Applications. Macromol Symp 240: 47-55.
- Kowalski A, Duda A, Penczek S (1998) Kinetics and mechanism of cyclic esters polymerization initiated with tin(ii) octoate, 1. Polymerization of e-caprolactone. Macromol Rapid Comm 19: 567-572.
- Grijpma DW, Pennings AJ (1991) Polymerization temperature effects on the properties of L-lactide and e- caprolactone copolymers. Polym Bull 25: 335- 341.
- Liu LZ, Ma HJ, Zhu XS, Jin ZH, Fan YJ (2008) Analysis of effect factors on the molecular weight of polylactide/nano-silica composites, Gaofenzi Cailiao Kexue Yu Gongcheng. Polym Mater Sci Eng 24: 63-72.
- Ipolitov EG, Artemov AB, Batrakov VV (2005) Physical Chemistry, Academy, Moscow.
- Du YJ, Lemstra PJ, Nijenhuis AJ, Huub van Aert AM, Bastiaansen C (1995) ABA Type Copolymers of Lactide with Poly(ethylene glycol). Kinetic, Mechanistic, and Model Studies. Macromolecules 28: 2124-2132.
- Zhang J (1999) Inferential estimation of polymer quality using bootstrap aggregated neural networks.Neural Networks12: 927-938.
- Senthil Kumar A, Ahmad Z (2011) Candida antarctica as catalyst for polycaprolactone synthesis: effect of temperature and solvents. Asia-Pac J Chem Eng 6: 398-405.
|
| |
| |