| Research Article |
Open Access |
|
| Takemi Otsuki1*, Suni Lee1, Naoko Kumagai-Takei1, Naomi Miyahara1, Minako Katoh1, Shoko Yamamoto1, Tamayo Hatayama1, Hidenori Matsuzaki1, Yasumitsu Nishimura1, Megumi Maeda1,2, Hiroaki Hayashi1,3, Junichi Hiratsuka4 and Ying Chen5 |
| 1Department of Hygiene, Kawasaki Medical School, 577 Matsushima, Kurashiki, 7010192, Japan |
| 2Department of Biofunctional Chemistry, Division of Bioscience, Okayama University, Graduate School of Natural Science and Technology, 3-1-1 Tsushima-naka, Okayama, 7008530, Japan |
| 3Department of Dermatology, Kawasaki Medical School, 577 Matsushima, Kurashiki, 7010192, Japan |
| 4Department of Radiation Oncology, Kawasaki Medical School, 577 Matsushima, Kurashiki, 7010192, Japan |
| 5Division of Pneumoconiosis, School of Public Health, China Medical University, 92 North 2nd, Heping District, Shenyang 110001, P. R. China |
| *Corresponding author: |
Takemi Otsuki
Department of Hygiene
Kawasaki Medical School
577 Matsushima
Kurashiki, 7010192, Japan
Tel: +81 86 462 1111
Fax: +81 86 462 1199
E-mail: takemi@med.kawasaki-m.ac.jp |
|
| |
| Received November 23, 2011; Published November 02, 2012 |
| |
| Citation: Otsuki T, Lee S, Kumagai-Takei N, Miyahara N, Katoh M, et al.(2012)Review of Reduced Tumor Immunity Caused by Asbestos Exposure to Immunocompetent Cells Such as T and NK Cells. 1:411. doi:10.4172/scientificreports.411 |
| |
| Copyright: © 2012 Otsuki T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Although the immunological effect of silica, SiO2, has been well investigated and involves the dysregulation of autoimmunity because of complications of autoimmune diseases found in silicosis, the immunological effects of asbestos, a mineral silicate possessing Si and O as core atoms, have not been well investigated. Asbestos can cause alteration of immunocompetent cells to result in a decline of tumor immunity. The experimental continuous exposure of chrysotile asbestos onto a human T cell line or NK cell line resulted in reduction of tumor immunity in both cell types. In the T cells, important molecules for tumor immunity such as CXCR3 chemokine receptor and IFN-γ exhibited reduced expression and production, respectively, not only for the cell line mode, but also for freshly isolated CD4 + T cells from healthy donors exposed ex vivo, as well as T cells derived from asbestos-exposed patients such as those with pleural plaque (PP) or malignant mesothelioma (MM). The activating receptors in NK cells such as NKG2D, 2B4 or NKp46 exhibited reduced expression in the asbestos-exposed NK cell line and freshly isolated NK cells exposed to asbestos ex vivo, from healthy donors as well as patients with PP or MM. Additionally, activation of the ERK signaling molecule is reduced in asbestos-exposed NK cells. These results strongly indicate asbestos exposure causes reduction of tumor immunity. Furthermore, improving these functions through certain bioactive substances in foods, plants or microorganisms may be a good tool for chemoprevention in asbestos- exposed people concerning future development of cancers. |
| |
| Keywords |
| |
| Asbestos; T cell; NK cell; Tumor immunity |
| |
| Introduction |
| |
| Asbestos exposure causes a variety of tumors such as malignant mesothelioma and lung cancers [1,2], in addition to laryngeal, gastrointestinal and bladder cancers [3-5]. |
| |
| Issues involving asbestos usage are also varied. Although the majority of developed countries have already banned the use of asbestos, a few nations are still exporting asbestos to developing countries and people in these asbestos-importing nations encounter many chances of being exposed to asbestos. Moreover, asbestos is still present in the buildings, water-pipes and other structures of nations that have even banned the material, and many workers such as the wrecking crews of buildings are under threat of attack from asbestos exposure. |
| |
| In Japan, the asbestos issue erupted in the summer of 2005 [6-8]. Residents were suddenly informed that asbestos, which was used in large amounts from the early 1950s up to the early 1990s in Japan with a maximum usage of approximately 352,000 tons in 1974, caused malignant mesothelioma (MM). Residents that lived near the asbestos handling manufacturer Kubota Corporation, in Amazasaki City, Hyogo Prefecture, developed MM. They had never worked in the asbestoshandling manufacture industry. In addition, medical information regarding MM induced anxiety in Japanese people, since the prognosis is very poor and there is no certain way to detect the cancer in the very early stage of the disease. Furthermore, people could not remember being exposed to asbestos 30 to 40 years ago. These matters increased the anxiety of residents. Furthermore, there is concern for people who are involved in processing earthquake rubble following Japan’s 2011 earthquake, since a news report revealed a case of mesothelioma for an individual who handled earthquake rubble after the Japanese Hanshin- Awaji earthquake in 1995 [9]. |
| |
| Reports of other issues regarding asbestos-induced malignant tumors such as malignant mesothelioma have not provided sufficient clinical, cellular or molecular details for an elucidation of these diseases in regard to better prevention, early diagnosis, and improvement of prognosis [10,11]. Of course, it is certain that recent advances in medical science regarding mesothelioma are progressing towards an understanding of the cellular and molecular aspects of mesothelioma cells that will contribute to newer diagnostic and therapeutic developments [12-14]. |
| |
| For example, the alteration of oncogenes and tumor suppressor genes in mesothelioma was recognized as a loss of p16-ink4 family cyclin dependent kinase-inhibitor (CDK-I) in the majority of cases of mesothelioma cells, and neurofibromin 2 (NF2)/merlin tumor suppressor was silenced by genomic deletion of epigenetic alteration in approximately half of the cases [15,16]. However, a large tumor suppressor homolog 2 (LATS2) gene located on chromosome 13q12 was recently reported as a newer tumor suppressor in mesothelioma. LATS2 encodes a serine/threonine kinase, a component of the Hippo tumor-suppressive signaling pathway localized down-stream of MF2 [17,18]. Transduction of LATS2 in mesothelioma cells with its mutation inactivated oncoprotein YAP, a transcriptional coactivator, via phosphorylation and inhibited cell growth. Moreover, it was reported that the BRCA1 (breast cancer susceptibility gene I) associated protein-1 (ubiquitin carboxy-terminal hydrolase)/BAP1 gene is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma [19,20]. |
| |
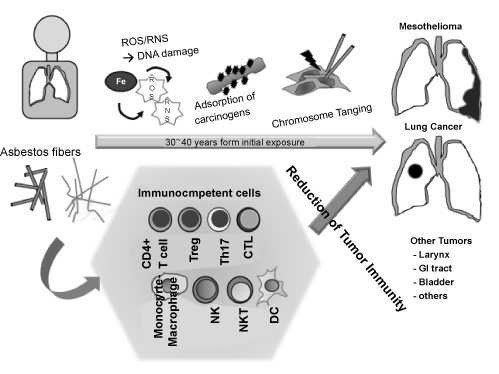
| On the other hand, the cellular and molecular effects of asbestos regarding carcinogenesis are considered to be caused by DNA damage due to production of reactive oxygen/nitrogen species (ROS/RNS) by iron-including asbestos [21,22], chromosome tangling due to direct physiological attack by asbestos fibers on cellular chromosomes, and adsorption of various carcinogens from cigarette smoking and air pollutants surrounding asbestos in the lung as shown in figure 1 [23-25]. |
| |
|
|
Figure 1: Schematic model showing mechanisms of carcinogenic activities of asbestos fibers, and the relationship of the immunological effects of asbestos in regard to chronic inflammation and reduced tumor immunity. |
|
| |
| We have been investigating the immunological effects of environmental substances such as silica and asbestos, which is a mineral silicate including SiO2 with magnesium, calcium and iron [26-28]. Silicosis patients suffer not only from pulmonary fibrosis, but often experience complications from autoimmune diseases [29,30] such as rheumatoid arthritis (known as Caplan’s syndrome) [31,32], Anti- Neutrophil Cytoplasmic Antibody (ANCA)-related vasculitis/nephritis [ 33,34], and systemic sclerosis [35,36]. Although the cause of this dysregulation of autoimmunity was thought to be due to the adjuvant effects of silica, we have demonstrated that alteration of CD95/Fas, the death receptor inducing cellular apoptosis, in silicosis patients [37-39] and direct activation of responder T cells and CD4+25+ forkhead box P3 (FOXP3) positive regulatory T (Treg) cells by silica exposure and resulting loss of Treg and imbalance of responder T cells and Treg causes reduction of the inhibitory effects of Treg against activation of responder T cells including auto-reacting T cells [40-43]. |
| |
| From these investigations, we thought that asbestos may affect the human immune system and that these effects would tend to reduce tumor immunity because of malignant complications in asbestosexposed patients. In this review, we introduce our experimental studies regarding asbestos exposure and alteration of tumor immunity using cell lines, freshly isolated human immunocompetent cells from healthy donors (HD), patients with pleural plaque (PP) who have a history of past exposure to asbestos, but not having malignancies, and patients with mesothelioma (MM) as shown in figure 1. |
| |
| Continuous and Low-Dose Exposure of Asbestos onto T Cells |
| |
| Temporary and high-dose exposure of chrysotile asbestos on a human HTLV-1 (Human Adult T Cell Leukemia Virus1) immortalized polyclonal T cell line, MT-2 |
| |
| First of all, we started to investigate the immunological effects of asbestos using a human T cell line and chrysotile. We used a cell line in order to further investigate cellular and molecular alteration of T cells, and chrysotile was selected because it was the most frequently used asbestos and resembled silica chemically. |
| |
| As we reported previously, a temporary and relatively high-dose (causing massive apoptosis for three days of in vitro exposure) exposure of chrysotile onto MT-2 cells caused apoptosis in a dose- and timedependent manner, activation of the mitochondrial apoptotic pathway involving phosphorylation of c-Jun N-terminal kinase (JNK) and p38 in the Mitogen-Activated Protein Kinase (MAPK) pathway, increase of BAX expression, release of cytochrome-c from mitochondria to the cytoplasm, and production of superoxide. These results indicated that asbestos can cause cellular and molecular alteration in lymphocytes similar to phenomena reported in pulmonary epithelial and mesothelial cells temporarily exposed to asbestos [44]. |
| |
| Continuous and low-dose exposure of chrysotile onto the MT-2 cell line |
| |
| After characterization of cellular and molecular alteration of MT-2 cells temporarily exposed to chrysotile, we attempted a continuous and relatively low-dose (doses causing less than half of cells to proceed to apoptosis when exposed temporarily) exposure experiment that was maintained for more than eight months. Thereafter, when a continuously exposed subline was released from chrysotile exposure and re-exposed to a high dose temporarily, it exhibited resistance to chrysotile-induced apoptosis. The cellular and molecular characterization of the subline revealed activation of Src-family kinases, upregulation of expression and secretion of interleukin (IL)-10, phosphorylation of Signal Transducer and Activator of Transcription 3 (STAT3), and upregulation of Bcl-2 localized down-stream of STAT3. These cascaded mechanisms were identified as cellular and molecular changes in MT-2 cells continuously exposed to chrysotile. In addition, other cellular changes involved upregulation of transforming growth factor (TGF)-β and reduced production of various cytokines. Furthermore, we established another five sublines that were exposed to chrysotile in a similar manner and independently. All of the six sublines exhibited similar gene expression profiles according to cDNA microarray analysis, and the effects on these cell lines are mostly confirmed as the effects of chrysotile on human T cells and not due to alterations obtained by chance. As further confirmation of these findings, serum TGF-β and IL-10 levels and the gene expression of Bcl-2 derived from peripheral CD4+ T cells from PP and MM patients exhibited increases of both cytokines and enhanced expression of Bcl-2 compared to HD [44,45]. |
| |
| Reduction of CXCR3 chemokine receptor and production of IFN-γ |
| |
| To investigate alteration of cellular and molecular markers in MT-2 sublines continuously exposed to chrysotile, the CXC chemokine receptor 3 (CXCR3) and interferon-γ were examined since cDNA array, pathway and signaling canonical analysis, as well as network analysis, indicated that these molecules were involved and altered in all sublines when compared with the original (never exposed) MT-2 line [46,47]. |
| |
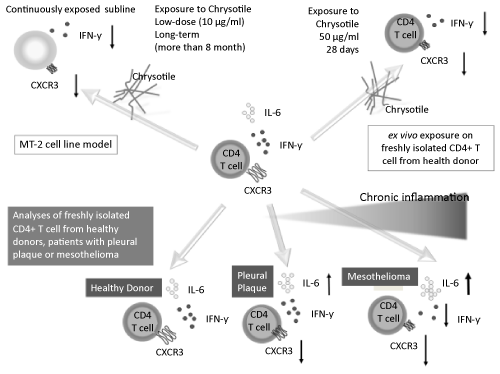
| Thus, the expression of CXCR3 in MT-2 sublines and in long-term exposure to chrysotile using freshly isolated peripheral CD4+ T cells were analyzed. All of the MT-2 sublines showed reduction of CXCR3 expression in mRNA and protein (examined via Western blotting, flow cytometry, and fluorescent immunohistological staining). In addition, four weeks of exposure to chrysotile caused reduction of CXCR3 expression when freshly isolated CD4+ T cells derived from six HDs were cultured with IL-2 and chrysotile. Similar to CXCR3, other Th1 type molecules such as IFN-γ, C-X-C motif chemokine 10 (CXCL10)/IFN- γ-induced protein 10 (IP-10) as the ligand for CXCR3, and chemokine (C-C motif) ligand 4 (CCL4)/Macrophage inflammatory protein-1 (MIP-1)-β exhibited reduced production or mRNA expression in all of the MT-2 sublines. In addition, the peripheral CD4+ T cells from patients with PP or MM exhibited increased IL-6 production compared with cells from HDs when these cells were stimulated in vitro with anti- CD3/CD28 antibodies for five days. Finally, peripheral CD4+ T cells derived from PP or MM patients showed CXCR3 surface expression [ 46,47]. |
| |
| Since CXCR3 is a G-protein-coupled seven-transmembrane receptor expressed on various lymphocytes including T, B and natural killer (NK) cells, it binds to IFN-γ-inducible chemokines such as CXCL9/ Monokine induced by IFN-γ(MIG), CXCL10/IP10 and CXCL11/IFNinducible T-cell alpha chemoattractant (I-TAC) that recruit leukocytes to inflammatory sites such as tumors. In the case of CD4+ T cells, the CXCR3 is preferentially expressed and IFN-γ-producing Th1/effector T cells exhibit a high-level production of inflammatory cytokines. As described above, there was a high production of anti-inflammatory cytokine IL-10 in MT-2 sublines and also in plasma from PP or MM patients. However, CD4+ T cells from these patients showed a high production potential of IL-6. Taken together, continuous exposure to asbestos caused reduction of tumor immunity induced by CXCR3 and IFN-γ cytokine networks, whereas the lesions where asbestos fibers are localized may be modified immunologically and there may be chronic inflammation suggested by potential IL-6 production and molecular changes leading to carcinogenesis. IL-10 may have an effect later when cells start to transform at the locus. As shown in figure 2, the overall results indicate that the effects of asbestos on CD4+ T cells modify the inflammatory and transforming status in the lesion, where asbestos and mesothelial cells are co-localized such that carcinogenesis develops slowly and progressively over 30 to 40 years, which is regarded as the latency period for the occurrence of mesothelioma [46-49]. |
| |
|
|
Figure 2: Schematic representation of asbestos-induced reduction of expression of a chemokine receptor, CXCR3, and expression and production of IFN-γ with increasing IL-6 using the MT-2 cell line model exposed continuously to a low-dose of chrysotile (upper left), an ex vivo exposure model using freshly isolated CD4+ T cells from healthy donors (HD) (upperright), as well as analyses of freshly isolated CD4+ T cells from HDs, and patients with pleural plaque (PP) and malignant mesothelioma (MM) (lower panels). |
|
| |
| Effects of asbestos on T Cells such as Treg |
| |
| The function and numbers of Treg are very important for a consideration of tumor immunity. As mentioned above, silica exposure can activate Treg to express the CD95/Fas molecule and induce early loss of Treg to create an imbalance between Treg and responder T cells which include self-antigen recognizing T cells. These effects may partially explain the occurrence of autoimmune diseases such as the complications of silicosis. |
| |
| We have been analyzing Treg functions in regard to asbestos using a continuous and low-dose exposure cell line or ex vivo stimulating models. However, we have so far not obtained results that have been confirmed, and will report the in vitro modification of Treg function using human cells in the near future. |
| |
| Alteration of NK cells Cytotoxicity and Expression of Activating Receptor |
| |
| Impairment of cytotoxicity of NK cells exposed to asbestos |
| |
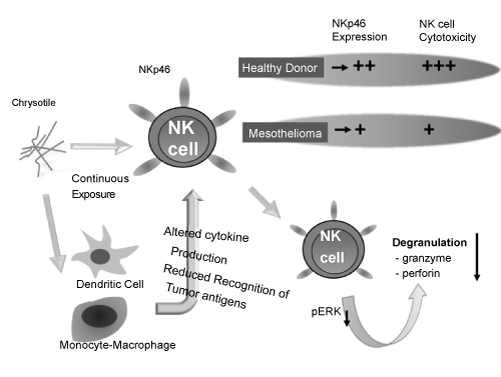
| Similar to the above-mentioned analyses targeting human T cells, the effects of asbestos on the function of NK cells, direct killers of tumor cells, were analyzed using a human NK cell line (YT-A1, kindly provided from Dr. Y. Yodoi, Kyoto University) and freshly isolated NK cells from HD exposed to chrysotile ex vivo. NK cell cytotoxicity was reduced when YT-A1 cells were continuously exposed to a relatively low-dose concentration (the same concentration used for the MT2 culture). Regarding cytotoxic granules, granzyme B and perforin exhibited reduced intracellular expression as analyzed by flowcytometry. Moreover, among the various NK cell-activating receptors, the expressions of NKG2d and 2B4, which belong to the NKG2 family characterized by a lectin-like domain, decreased during continuous exposure. In addition, freshly isolated NK cells from HDs exposed ex vivo showed the same reduction of cytotoxicity, whereas the key molecule influenced by chrysotile exposure was NKp46-activating receptor belonging to the NCR (natural cytotoxicity receptor) group with NKp44 and NKp30. Furthermore, freshly isolated NK cells from patients with MM exhibited a reduction of NKp46, but not NKG2D or 2B4. Taken together and considering that the results obtained from the cell line may not be natural, the target of asbestos that reduces NK cell cytotoxic activity is the NKp46 molecule. It may be possible to use the expression level of NKp46 in peripheral NK cells as a marker for asbestos-exposure or tumor-bearing in people who have handled asbestos through the work environment or those who lived near asbestos-handling industries as shown in figure 3 [50]. |
| |
|
|
Figure 3: Schematic representation of the effects of asbestos exposed directly onto NK cells or indirectly via through dendritic cells or monocytemacrophage lineage cells, reduction of NK cell activating receptor, NKp46, with the relationship among disease status as represented by HD, PP or MM, and reduction of ERK1/2 phosphorylation and degranulation in NK cells with cytotoxicity reduced by asbestos exposure. |
|
| |
| Signaling disturbance in NK cells exposed to chrysotile |
| |
| |
| As mentioned above, NK cell activity was reduced by asbestos exposure. Alteration of the signaling pathway was analyzed according to the reduction of NKG2D and 2B4 expression levels in the NK cell line we used. Although NK cell-activating receptors possess different adaptor molecules to induce degranulation, the most important among these molecules is extracellular signal-regulated kinase (ERK). Thus, the phosphorylation status of ERK1/2 was examined using the YT-A1 subline continuously exposed to chrysotile. In spite of the enhancement of ERK1/2 phosphorylation when YT-A1 original cells were cultured with target tumor cells, the K562 subline did not show any increase of phosphorylation of ERK1/2. In addition, the reduction of phosphorylation of ERK1/2 in the YT-A1 cell line was also obtained when cells were treated with wortomannin and PP2, inhibitors of phosphoinositide 3-kinase (PI3K) and Src-family kinase, respectively, and when NK cells were cultured with target K562 cells and anti-2B4 or NKp46 antibodies were added to the culture [51]. |
| |
| These results strongly suggest that NK cells exposed to asbestos reduced their cytotoxic activity via inhibition of signaling pathways involving the ERK1/2 molecule and decrease of degranulation in perforin and granzyme B as shown in figure 3. Further investigation will be conducted regarding modification of NK cell activity influenced by asbestos-exposed dendritic cells or monocyte/macrophage lineage cells, since their production of cytokines may affect K cell activity and production may be altered by asbestos exposure onto these types of cells [50-52]. |
| |
| Conclusion |
| |
| As described above, the effects of asbestos on T cells and NK cells reduce their activity against tumor cells. However, other immunocompetent cells such as Treg, Th17, CD8+ cytotoxic T cells (CTL), and natural killer T (NKT) cells, as well as antigen presenting cells such as D, may influence their function following continuous exposure to asbestos. Alteration of the functions and differentiations of these various cells affected by asbestos exposure should be investigated to better understand the reduction of tumor immunity caused by chronic asbestos exposure. In particular, the effects on Treg, CTL and Th17 cells should be analyzed immediately and it should be determined how asbestos influences total tumor immunity and how strongly individual cell type is involved in reduced tumor immunity. Moreover, the effects of other fibers such as crocidolite and amosite should be investigated since these iron-including fibers are thought to possess stronger carcinogenicity than chrysotile. |
| |
| The processes involved in tumor immunity in asbestos-exposed people should be elucidated for the purpose of chemoprevention of asbestos-induced malignancies. Some bioactive substances including foods, plants or microorganisms may recover the reduced function of immunocompetent cells caused by asbestos-exposure. These types of trials may help to relieve the anxiety experienced by most people who worked in the asbestos-handling industries or lived near these manufacturers. |
| |
| Acknowledgement |
| |
| The authors specially thank Ms. Yoshiko Yamashita, Tomoko Sueishi, Keiko Kimura, and Misao Kuroki for their technical help. The experimental results performed by us and presented partly in this review were supported by Special Funds for Promoting Science and Technology (H18-1-3-3-1), JSPS KAKENHI (22790550, 22700933, 20390178, 20890270, 19689153, 19790431, 19790411, 18390186, 16390175 and 09670500), the Takeda Science Foundation (Tokutei Kenkyu Josei I, 2008), and Kawasaki Medical School Project Grants (22-B1, 22- A58, 22-A29, 21-401, 21-201, 21-107, 20-411I, 20-210O, 20-109N, 20-402O and 20-410I). |
| |
| |
| References |
| |
- Mossman BT, Lippmann M, Hesterberg TW, Kelsey KT, Barchowsky A, et al. (2011) Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J Toxicol Environ Health B Crit Rev 14: 76-121.
- Alberg AJ, Brock MV, Samet JM (2005) Epidemiology of lung cancer: looking to the future. J Clin Oncol 23: 3175-3185.
- Craighead JE, Gibbs AR (2008) Asbestos and Its diseases. Oxford University Press, Inc, New York, USA.
- Rolston R, Oury TD (2004) Other Neoplasia: Pathology of asbestos-associated diseases. (2nd edn), Springer Science+Business Media Inc, New York, USA.
- Freidman GK (2005) Clinical diagnosis of asbestos-related diseases: Asbestos: risk assessment, epidemiology, and health effects. Taylor & Francis, CRC Press, New York, USA.
- Kumagai S, Kurumatani N (2009) Asbestos fiber concentration in the area surrounding a former asbestos cement plant and excess mesothelioma deaths in residents. Am J Ind Med 52: 790-798.
- Kurumatani N, Kumagai S (2008) Mapping the risk of mesothelioma due to neighborhood asbestos exposure. Am J Respir Crit Care Med 178: 624-629.
- Kumagai S, Kurumatani N, Tsuda T, Yorifuji T, Suzuki E (2010) Increased risk of lung cancer mortality among residents near an asbestos product manufacturing plant. Int J Occup Environ Health 16: 268-278.
- Nukushina J (1995) Japanese earthquake victims are being exposed to high density of asbestos. We need protective masks desperately. Epidemiol Prev 19: 226-227.
- Campbell NP, Kindler HL (2011) Update on malignant pleural mesothelioma. Semin Respir Crit Care Med 32: 102-110.
- Jamrozik E, de Klerk N, Musk AW (2011) Asbestos-related disease. Intern Med J 41: 372-380.
- Heintz NH, Janssen-Heininger YM, Mossman BT (2010) Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. Am J Respir Cell Mol Biol 42: 133-139.
- Yano S, Li Q, Wang W, Yamada T, Takeuchi S, et al. (2011) Antiangiogenic therapies for malignant pleural mesothelioma. Front Biosci 16: 740-748.
- Bagia M, Nowak AK (2011) Novel targeted therapies and vaccination strategies for mesothelioma. Curr Treat Options Oncol 12: 149-162.
- Sekido Y (2010) Genomic abnormalities and signal transduction dysregulation in malignant mesothelioma cells. Cancer Sci 101: 1-6.
- Musti M, Kettunen E, Dragonieri S, Lindholm P, Cavone D, et al. (2006) Cytogenetic and molecular genetic changes in malignant mesothelioma. Cancer Genet Cytogenet 170: 9-15.
- Sekido Y (2011) Inactivation of Merlin in malignant mesothelioma cells and the Hippo signaling cascade dysregulation. Pathol Int 61: 331-344.
- Murakami H, Mizuno T, Taniguchi T, Fujii M, Ishiguro F, et al. (2011) LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res 71: 873-883.
- Testa JR, Cheung M, Pei J, Below JE, Tan Y, et al. (2011) Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 43: 1022-1025.
- Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, et al. (2011) The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 43: 668-672.
- Liu G, Beri R, Mueller A, Kamp DW (2010) Molecular mechanisms of asbestos-induced lung epithelial cell apoptosis. Chem Biol Interact 188: 309-318.
- Kamp DW (2009) Asbestos-induced lung diseases: an update. Transl Res 153: 143-152.
- Nagai H, Toyokuni S (2010) Biopersistent fiber-induced inflammation and carcinogenesis: lessons learned from asbestos toward safety of fibrous nanomaterials. Arch Biochem Biophys 502: 1-7.
- Toyokuni S (2009) Mechanisms of asbestos-induced carcinogenesis. Nagoya J Med Sci 71: 1-10.
- Toyokuni S (2009) Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci 100: 9-16.
- Maeda M, Nishimura Y, Kumagai N, Hayashi H, Hatayama T, et al. (2010) Dysregulation of the immune system caused by silica and asbestos. J Immunotoxicol 7: 268-278.
- Otsuki T, Maeda M, Murakami S, Hayashi H, Miura Y, et al. (2007) Immunological effects of silica and asbestos. Cell Mol Immunol 4: 261-268.
- Otsuki T, Hayashi H, Nishimura Y, Hyodo F, Maeda M, et al. (2011) Dysregulation of autoimmunity caused by silica exposure and alteration of Fas-mediated apoptosis in T lymphocytes derived from silicosis patients. Int J Immunopathol Pharmacol 24: 11S-16S.
- Steenland K, Goldsmith DF (1995) Silica exposure and autoimmune diseases. Am J Ind Med 28: 603-608.
- Uber CL, McReynolds RA (1982) Immunotoxicology of silica. Crit Rev Toxicol 10: 303-319.
- Caplan A (1959) Rheumatoid disease and pneumoconiosis (Caplan's syndrome). Proc R Soc Med 52: 1111-1113.
- Caplan A, Payne RB, Withey JL (1962) A broader concept of Caplan's syndrome related to rheumatoid factors. Thorax 17: 205-212.
- Saeki T, Fujita N, Kourakata H, Yamazaki H, Miyamura S (2004) Two cases of hypertrophic pachymeningitis associated with myeloperoxidase antineutrophil cytoplasmic autoantibody (MPO-ANCA)-positive pulmonary silicosis in tunnel workers. Clin Rheumatol 23: 76-80.
- Bartůnková J, Pelclová D, Fenclová Z, Sedivá A, Lebedová J, et al. (2006) Exposure to silica and risk of ANCA-associated vasculitis. Am J Ind Med 49: 569-576.
- Haustein UF, Ziegler V, Herrmann K, Mehlhorn J, Schmidt C (1990) Silica-induced scleroderma. J Am Acad Dermatol 22: 444-448.
- Rustin MH, Bull HA, Ziegler V, Mehlhorn J, Haustein UF, et al. (1990) Silica-associated systemic sclerosis is clinically, serologically and immunologically indistinguishable from idiopathic systemic sclerosis. Br J Dermatol 123: 725-734.
- Otsuki T, Miura Y, Nishimura Y, Hyodoh F, Takata A, et al. (2006) Alterations of Fas and Fas-related molecules in patients with silicosis. Exp Biol Med (Maywood) 231: 522-533.
- Tomokuni A, Aikoh T, Matsuki T, Isozaki Y, Otsuki T, et al. (1997) Elevated soluble Fas/APO-1 (CD95) levels in silicosis patients without clinical symptoms of autoimmune diseases or malignant tumours. Clin Exp Immunol 110: 303-309.
- Otsuki T, Sakaguchi H, Tomokuni A, Aikoh T, Matsuki T, et al. (1998) Soluble Fas mRNA is dominantly expressed in cases with silicosis. Immunology 94: 258-262.
- Wu P, Miura Y, Hyodoh F, Nishimura Y, Hatayama T, et al. (2006) Reduced function of CD4+25+ regulatory T cell fraction in silicosis patients. Int J Immunopathol Pharmacol 19: 357-368.
- Hayashi H, Miura Y, Maeda M, Murakami S, Kumagai N, et al. (2010) Reductive alteration of the regulatory function of the CD4(+)CD25(+) T cell fraction in silicosis patients. Int J Immunopathol Pharmacol 23: 1099-1109.
- Kumagai N, Hayashi H, Maeda M, Miura Y, Nishimura Y, et al. (2011) Immunological effects of silica and related dysregulation of autoimmunity: Autoimmune Disorder / Book 1. InTech Open Access Publisher, Croatia (in press).
- Hayashi H, Nishimura Y, Hyodo F, Maeda M, Kumagai N, et al. (2011) Dysregulation of autoimmunity caused by silica exposure: Fas-Mediated apoptosis in T lymphocytes derived from silicosis patients: Autoimmune Disorders: Symptoms, Diagnosis and Treatment. Nova Science Publishers, Inc., Hauppauge, NY, USA.
- Hyodoh F, Takata-Tomokuni A, Miura Y, Sakaguchi H, Hatayama T, et al. (2005) Inhibitory effects of anti-oxidants on apoptosis of a human polyclonal T-cell line, MT-2, induced by an asbestos, chrysotile-A. Scand J Immunol 61: 442-448.
- Miura Y, Nishimura Y, Katsuyama H, Maeda M, Hayashi H, et al. (2006) Involvement of IL-10 and Bcl-2 in resistance against an asbestos-induced apoptosis of T cells. Apoptosis 11: 1825-1835.
- Miura Y, Nishimura Y, Maeda M, Murakami S, Hayashi H, et al. (2008) Immunological alterations found in mesothelioma patients and supporting experimental evidence. Environ Health Prev Med 13: 55-59.
- Maeda M, Nishimura Y, Hayashi H, Kumagai N, Chen Y, et al. (2011) Reduction of CXC chemokine receptor 3 in an in vitro model of continuous exposure to asbestos in a human T-cell line, MT-2. Am J Respir Cell Mol Biol 45: 470-479.
- Maeda M, Nishimura Y, Hayashi H, Kumagai N, Chen Y, et al. (2011) Decreased CXCR3 expression in CD4+ T cells exposed to asbestos or derived from asbestos-exposed patients. Am J Respir Cell Mol Biol 45: 795-803.
- Kumagai-Takei N, Maeda M, Chen Y, Matsuzaki H, Lee S, et al. (2011) Asbestos induces reduction of tumor immunity. Clin Dev Immunol 2011: 481439.
- Matsuzaki H, Maeda M, Lee S, Nishimura Y, Kumagai-Takei N, et al. (in press) Asbestos-induced cellular and molecular alteration of immunocompetent cells and the relationship with chronic inflammation and carcinogenesis. J Biomed Biotechnol.
- Nishimura Y, Miura Y, Maeda M, Kumagai N, Murakami S, et al. (2009) Impairment in cytotoxicity and expression of NK cell- activating receptors on human NK cells following exposure to asbestos fibers. Int J Immunopathol Pharmacol 22: 579-590.
- Nishimura Y, Maeda M, Kumagai N, Hayashi H, Miura Y, et al. (2009) Decrease in phosphorylation of ERK following decreased expression of NK cell-activating receptors in human NK cell line exposed to asbestos. Int J Immunopathol Pharmacol 22: 879-888.
|
| |
| |