|
|
| Pravin N Lambade1*, R.S.Dolas2, Neena Virani1 and Dipti P Lambade3 |
| 1Department of Oral and Maxillofacial Surgery, Swargiya Dadasaheb Kalmegh Smruti Dental College and Hospital, Nagpur, India |
| 2Department of Oral and Maxillofacial Surgery, Sarswati Dental College, Lucknow, U.P, India |
| 3VSPM Dental College, Nagpur, MH, India |
| *Corresponding author: |
Pravin N Lambade
Professor
Department of Oral and Maxillofacial Surgery
Swargiya Dadasaheb Kalmegh Smruti Dental College and Hospital
Nagpur, India
Tel: +91-09822236370
E-mail: drpravinlambade@gmail.com |
|
| |
| Received February 12, 2012; Published November 02, 2012 |
| |
| Citation: Lambade PN, Dolas RS, Virani N, Lambade DP (2012) Cervicofacial Necrotising Fasciitis of Odontogenic Origin: A Review. 1:414. doi:10.4172/scientificreports.414 |
| |
| Copyright: © 2012 Lambade PN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Introduction |
| |
| A ‘Killer or flesh eating Bug’ as known to the Military surgeon of past centuries [1] was due to its nature of rapid spread along the facial planes to involve the sub cutaneous tissues, fascia, skin and possibly muscle[ 1,2]. Copious amount of foul-smelling pus are typically produced. Blood vessels become thrombosed, and ultimately tissue death occurs [1,3]. Now well known as Necrotizing fasciitis, due to presence of innumerable data, has become a common complication as an exacerbation of pre-existing infection. |
| |
| Historical Background |
| |
| The Evidence of this disease can be traced back to Hippocrates in his works where he described it as “Sometimes a very small wound broke out and if such an accident was neglected great inflammation took place. In most of them the abscess ended in suppuration and there was great failing off of the flesh, tendons and bones; and the defluxion which seated in the parts was not like pus, but a sort of putrefaction and the running was large and of various characters (Figures 1 and 2). About the head these things were accompanied by falling off of the hairs of the head and chin; the bones were laid bare and separated and there were excessive running; and these symptoms happened in fevers and without fevers” [4]. |
| |
|
|
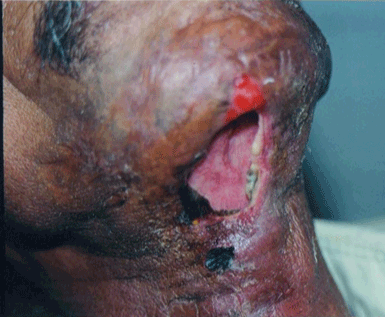
Figure 1: Necrotising Fascitis in Diabetic Patient. |
|
| |
|
|
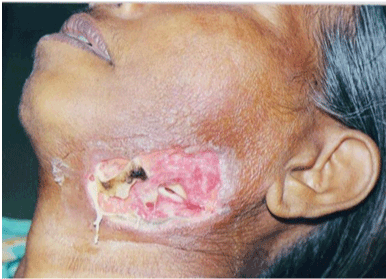
Figure 2: Necrotising Fascitis Secondary to Ludwigs Angina. |
|
| |
| Since then Vague clinical descriptions of the disease process that lack diagnostic details have been found in historical texts; however, necrotising fasciitis was clearly described in late eighteenth century by Claude Pouteau, chief surgeon in the Hotel Dieu in Lyon in 1783 [5]. At that time the disease complex was given many names, such as “malignant ulcer”, ”gangrenous ulcer”, “phagedenis ulcer”, “putrid ulcer”, “Phagedena gangraenosa” and “hospital gangrene” [5]. |
| |
| In the late eighteenth century, a series of outbreaks affected the British Home Fleet. In confined quarters the disease could spread quickly. On HMS San Josef the surgeon observed ‘‘. . . an ulcer that had devoured one side of a sailor’s face, which had followed a blow on the ear, that was attended by a very slight wound’’ [6]. In the early nineteenth century, the disease was reported from military hospitals by the name of ‘‘hospital gangrene’’ or ‘‘Phagedena gangraenosa’’. In one case, “half the cranium was denuded, the bones having become as black as charcoal; in another the neck was denuded to expose the trachea”[5]. |
| |
| The disease was recorded in the Gendarmerie Hospital at Brussels after Waterloo [5], and Miss Nightingale noted 80 cases in 1 month at Scutari [5]. The disease was well known to the surgeons in the American Civil War, and Joseph Jones (Confederate Army surgeon) is credited with the first clear investigation and characterization of hospital gangrene [5]. This disease as sever in its form was also considered fatal. A serious outbreak occurred in September 1862 in the hospitals at Fredrick and West Philadelphia after the battles of South Mountain and Antietam. In January 1863, because of the poor prisoner of war sanitation in Richmond, three outbreaks occurred as the sick were transferred to Annapolis. |
| |
| Similar kind of disease process was described as genital disease confined to prostitutes and the destitutes that were admitted in St Bartholomew’s Hospital London in around 1844, where the disease was aggressive and if left unchecked “…involves in its ravages the vagina, perineum and anus and sometimes even the bladder and uterus” [5]. |
| |
| Fournier’s classical description of the condition was of Phagedena of the penis and scrotum, but case histories demonstrated that it was the same disease process as reported by the military surgeons [6]. Sporadic cases continued to be reported from across the continents into the early twentieth century. An American surgeon reported a hospital outbreak in Peking, where it was more common than in the West [7]. Melaney for the first time isolated a hemolytic Streptococcus from the wounds, and his name subsequently was associated with the disease (Melaney’s gangrene). In 1952, Wilson coined the name ‘‘necrotizing fasciitis,’’ which described the main feature of the disease and emphasized the polymicrobial nature of some of the infections [ 2,8,9]. More contemporary reviews of cervical necrotising fasciitis of head and neck were published by Spankus et al. [9], Barcerak et al. [ 10], and Banerjee et al. [11]. As the disease became more known in medical faculty, an exhausting review has been done by Mcurk in 2003 regarding the diagnosis and treatment of NF in head and neck region [ 5]. Currently, it occurs as unexpected isolated attacks so that few oral and maxillofacial surgeons have any experience with it. The effects remain as devastating as ever if not checked. |
| |
| Microbiology |
| |
| Over the centuries extensive studies have been done in separating various organisms associated with NF. For a long time group A Streptococus were considered to be the principal causative organism related to NF. But as culture techniques improved, it is now clear that most necrotizing wounds sustain a mixture of bacteria and sometimes fungus [12] working synergistically. Various bacterial strains may dominate different wounds, but essentially necrotizing fasciitis may be categorized into three types according to the causative organism. (i) In cool and temperate climates it tends to be associated with group A b-haemolytic streptococci (Streptococcus pyogenes) [13] alone or with Staphylococcus aureus. They are the only bacteria that seem to be able to generate solely this clinical picture. Serotypes M1 and M3 are the most common S. pyogenes serotypes associated with invasive disease [14], but multilocus sequence typing done by Enright et al. confirmed the genetically diverse range of strains in association with these infections [ 15]. (ii) In many cases (up to 60%) the necrotizing fasciitis may be polymicrobial, including one or more obligate anaerobes [13,16]. Brook and Frazier [17] reviewed 87 cases of necrotizing fasciitis over a 17-year period. Of these cases, only 4 were monoinfections with S. pyogenes. In the remaining cases, anaerobic bacteria were predominant, with Peptostreptococcus, Prevotella, Porphyromonas, Bacteroides, and Clostridium the commonest genera isolated. Facultative anaerobic bacteria, such as Enterobacteria, are also important. Up to 11 bacterial species have been cultured with various streptococci (groups B and F) in attendance, not just group A [17]. (iii) In tropical climates, the condition can be caused by members of the family Vibrionacae, which are of seawater origin [5]. In Colombia, the dominant genus was a mycosis that was particularly virulent and proved lethal in 70% of cases [ 18]. In India, a type of mucormycosis was isolated in 18 cases of NF from 1998 to 2004. [12] |
| |
| Streptococcus pyogenes produce several virulence factors that are likely to be involved in necrotizing fasciitis, including the extracellular pyrogenic exotoxins A, B, and C together with other exotoxins and superantigens [5]. Given this powerful virulence armory, it is perhaps surprising that invasive S. pyogenes infections are relatively rare. One reason for this might be the demonstration that mutations in the two-component CsrS/CsrR 2-component regulatory system led to increased virulence in a mouse model [18]. It could be hypothesized that exotoxin production by S. pyogenes is normally tightly regulated but that when that control is lost through mutation in the regulatory gene, a hypervirulent phenotype results. The pathogenesis of the polymicrobial form of the infection is unclear, although it is well known that consortia of bacteria work together to evade the host defences and cause tissue damage. Host factors also predispose to the rapid spread of some infections [5]. |
| |
| Pathogenesis |
| |
| The real trigger for any minor injury to develop into NF is still disputed, but many studies show that, patients who are immunologically compromised by either medical therapy, Diabetes mellitus, HBsAg, HIV, or any other debilitating conditions are more susceptible to cervical necrotising fasciitis. In a case reported by Shraddha Jain et al., anaemia and chronic alcoholism has also been shown to be the predisposing factor to develop NF post trauma [19]. Lack of personal hygiene especially in rural areas, or close quarters of Army camps during civil wars where hygienic conditions were difficult to maintain have also been the trigger factors for NF [4]. |
| |
| In early stages NF in head and neck region is often misdiagnosed as erysipelas or cellulitis [11,12,20]. Patients are typically febrile, tachycardic, and dehydrated due to dysphagia secondary to neck edema or trismus [10,11,20]. Mandibular second or third molars are frequently the source of odontogenic infections that proceed to cervical NF [21]. |
| |
| The overarching feature of necrotizing fasciitis is a rapid, progressive liquefaction of the subcutaneous fat and connective tissue below a relatively normal looking skin surface [5]. Some studies show overlying skin to be erythematous and tense and may be hyperesthetic or anesthetic to touch [10]. The disease is usually confined to the sub cutaneous tissues and rarely involves muscle [2,8,22]. The fascial planes disintegrate, and with the ensuing necrosis come edema and the release of tissue fluid. Early in the development of the disease the veins that traverse the liquefying subdermal fat become inflamed and start to thrombose, which gives the skin first a red and then a mottled color. Later the arterial supply is also jeopardized and the skin becomes pale, which leads to necrosis and wet (coliquative) gangrene. The bacteria initiate an acute local inflammatory response within the dermis that is characterized by an intense polymorphonuclear infiltrate, focal necrosis, and micro abscess formation. The histologic picture is one of arteriolar and venous thrombosis of the subcutaneous fat, whereas the adjoining muscle shows comparatively little inflammation [5]. The area is acutely painful, and the surrounding tissues are red (the signs depend on the specific mix of bacteria), but on close inspection a central portion of skin is pale and toxic. The skin subsequently develops a slightly mottled appearance as it becomes congested through venous stasis. As the perfusion is further reduced through arterial failure the skin starts to blister. Sensory perception is lost as nerves are destroyed and the wound weeps fluid from the underlying liquefaction [5]. Gross edema is a feature of the disease, and gas may be present in up to 40% of cases [17]. |
| |
| Clinical Features |
| |
| The incidence of this disease increases with age (median age, 57 years) and most adult cases (70%) occur in patients with at least one underlying chronic illness (immunosuppression, diabetes, alcohol/drug abuse, malignancy, or chronic systemic disease). Children by contrast tend not to have chronic illness, but necrotizing fasciitis may complicate chickenpox. Occasionally the disease afflicts apparently healthy individuals. It has been suggested (unconvincingly) that antinflammatory medication might predispose to these spreading infections by interfering with granulocyte function [23]. |
| |
| In early stages, the patient complaints of pain about head and neck region with rapid exacerbation of symptoms. Dysphagia, odynophagia, increasing pain, trismus, paraesthesia, and sometimes dyspnea are typically present [24]. If there are systemic symptoms of generalised malaise or tachycardia in the presence of an apparently innocuous wound, however, necrotizing fasciitis should be considered early [5]. The skin, in initial stages, becomes hot, smooth, tense, shiny due to the underlying edema and painful without a clear demarcation between normal and affected skin [24]. In later stage, shows a rapidly enlarging swelling about the head and neck, with 3 zones of demarcation: a wide peripheral zone of erythema outside a zone of tender purple skin surrounding a central black necrotic area [9]. Fetid odour being the hallmark of dead tissue is always present. Crepitations maybe felt suggestive of gas formation. The presence of gas is neither a reliable nor discriminatory sign for clostridial infection because it can be absent in gas gangrene and present in various nonclostridial infections [16]. Gas simply denotes the presence of anaerobic bacteria [25]. |
| |
| Management |
| |
| Two treatments are suggested by Gurk [5] 1) Surgery 2) Antibiotics with HBO therapy. Almost all the studies reported suggest a rapid and radical surgical management of NF. Time is of essence, because mortality is associated with delayed intervention. Surgical debridement and fasciotomy within 24 hours have proved very helpful in reducing morbidity and mortality rates [5]. Management is similar to that of an extensive burn. Gurk suggests the wounds should be washed (hydrogen peroxide is useful for debridement) and packed regularly (every 4 hours) Underlying muscle can be preserved, but all necrotic tissue and overlying skin must be removed [5]. Resected tissue (skin, muscle, connective tissue) should be sent for culture and antibiotic sensitivity (aerobic and anaerobic), and Gram’s stain results should be obtained, a procedure best done personally by the attending surgeon. Slowly the slough clears and shiny granulation tissue emerges from beneath the yellow slime. The undermined skin at the edge of the wound reattaches to the underlying granulation tissue and the packing can be withdrawn slowly day by day. Regular dressing still should be maintained at 8-hour intervals, which demands a heavy nursing commitment. |
| |
| Necrotic skin is removed followed by cosmetic correction with split skin graft, at later stage [19]. Antibiotic regimen should be altered to provide maximum specific coverage to combat the offending pathogens once the culture and sensitivity results are known. HBO therapy has been gaining support in management of NF. Recently published reports show a 50% reduction in mortality when HBO is used along with surgery to treat NF [22,26,27]. |
| |
| Shock and multiorgan failure are relatively common, so resuscitation and general supportive measures are vital in the established case. Airway maintenance is a primary concern in cervical NF patients. Neck edema and necrosis increase difficulty in intubation for which a planned tracheostomy could be considered [5]. |
| |
| Discussion |
| |
| It is now an established fact that, systemic symptoms in presence of an apparently innocuous wound, necrotising fasciitis should be considered early. NF has typical characteristic features of: |
| |
| • Extreme rapidity with which the disease progresses, distinguishes it from standard gangrene. |
| |
| • A tendency to turn subcutaneous tissues into putrid, pulpy substance. |
| |
| • Sever pain together with a smell which is peculiar and extremely offensive. |
| |
| • Starts at the site of any small wound or scratch, attacking mainly immunologically compromised patients but also seen in healthy adults. Unhygienic surroundings also play an important role in establishing NF [5]. |
| |
| The rate of necrosis is disproportionate to the signs and symptoms of infection. A small wound can be painful [5]. Early systemic symptoms may be subtle and amount to little more than a feeling of malaise or tachycardia. The condition even has been reported after routine dental surgery or dental sepsis. The variable clinical picture means that delay in diagnosis is common, because the prodromal period in which the synergistic consortia of bacteria are evolving may be only 3 or 4 days before the phase of rapid acceleration. Diagnosis depends on being alert to the possibility of the disease and recognizing the pattern of clinical events, the main feature of which is a rapidly progressive necrotizing infection [5]. |
| |
| In the initial stages, before necrosis is seen, the infection spreads in the subcutaneous tissues and may appear as a routine odontogenic deep neck space abscess. Rapid spread could be attributed to the lack of integrity of subcutaneous fat offering little resistance. Delay in diagnosis leads to increase in the area of necrosis, with a resulting increase in cosmetic deformity and life-threatening complications. The condition can result from dental (dental abscess, gingivitis, pulpitis, etc.), [28,29] sinus, [30] peritonsillar, [31,32] and salivary gland [26] infections, or from infections secondary to surgery, [33] insect bites, or trauma [34]. Dental infections are the most common etiologic factor, followed by trauma, peritonsillar and pharyngeal abscesses, and osteoradionecrosis. |
| |
| Medically compromised patients lack the ability to combat basic infection and thus are the main targets for developing NF in a relatively minor infection. An extensive review done by Lee et al. [24] in 2000 discuss the fact that, hyperglycaemia impairs leukocyte function and contributes to host immune system, thus, rendering the diabetic more susceptible to develop NF. Malnourished patients, due to their decreased number of circulating lymphocytes and T cells, as well as an impaired antibody response and polymorphonuclear cell function, increase their chance of developing NF [24]. |
| |
| Compromised circulatory system in obese individuals and with peripheral vascular disease patients, render them susceptible to NF. Similar is the case with impaired liver function in Hepatitis and cirrhosis. In HIV, the drastic fall of immunity renders patient more susceptible. |
| |
| Radiological study is useful to evaluate the airway status in cervical NF. In early stages, however, standard radiographs provide minimum information. Once the disease has progressed sufficiently, small opacities in soft tissue representing gas formation can be observed [ 22,35]. De Backer et al. [22] showed consistent findings on CT scan of cellulitis, fasciitis, myositis, and multiple space fluid collection, mediastinal fat and mediastinal fluid collection, pleural and pericardial effusions. Saiag et al. did MRI scans that suggested “numerous well defined dome shaped areas of hypersignal in the deep hypodermis on T2-weighted images” [36]. |
| |
| In case of compromised airway, a planned tracheostomy by skilled surgeon in operation theatre is preferable and advisable by many authors, as it provides secure airway throughout the hospital stay [24]. More than 50% of patients develop significant hypotension. In 10% to 30% of cases the disease is complicated by one or more of the following conditions: acute renal failure, coagulopathy, abnormal liver function, acute respiratory distress syndrome, or hemolytic anemia. The rapid progression of the disease is its distinguishing feature [5]. |
| |
| After surgical management HBO therapy is popular as an adjunctive treatment [22,26,27]. Recently published reports show 50% reduction in mortality rate and about 20 day reduction in hospital stay when HBO is used [27,37]. The many benefits of HBO therapy such as, enhanced lysosomal degradation potential, formation of free radicals of oxygen that are cidal to many anaerobes, elevated tissue partial pressures of oxygen that inactivates many exotoxins produced by microorganisms, temporary reversal of tissue hypoxia and its main advantage of neoangiogenesis. This advantage of neovascularization helps to preserve the remaining tissue as well as form an adequate bed of granulation tissue on which subsequent reconstruction can be based. |
| |
| In developing countries like India there are very few centres where HBO therapy is given and also because of economic constraints all patients cannot afford to go for this therapy, however, still much can be achieved by immediate surgical management , and culture report guided antibiotics adjustments. The key is early diagnosis and stopping the infection right at its infant stage. As per Indian Mythology suggests , that has become true in todays time, “ Many huge giants can be send to dust by proper planning, approach and lots of patience.” |
| |
| |
| References |
| |
- Deans M (1994) Flesh-eating bugs scare. Lancet 343: 1418.
- Rapoport Y, Himelfarb MZ, Zikk D, Bloom J (1991) Cervicofacial Necrotizing Fascitis of Odontogenic Origin. Oral Surg Oral Med Oral Patho 72: 15-18.
- McAndrew P G, Davis SJ, Griffiths RW (1987) Necrotizing fasciitis caused by dental infection. Br J Oral Maxillofac Surg 25: 314-322.
- Adams F (1785) The genuine work of Hippocrates. London Medical Journal 6: 373-400.
- Gurk MM (2003) Diagnosis and treatment of necrotizing fasciitis in the head and neck region. Oral maxillofac surg clin north Am 15: 59-67.
- Travers T (1824) Two cases of slough ulceration. London Medical and Physical Journal 1824: 122-134.
- Melaney FL (1924) Hemolytic Streptococcus gangrenehemolytic. Arch Surg 9: 317-364.
- Green RJ, Dafoc DC, Rattin TA (1996) Necrotizing Fascitis. Chest 110: 219-229.
- Spankus EM, Flint PW, Smith RJ, Miller RH (1984) Craniocervical necrotizing fasciitis. Otolaryngol Head Neck Surg; 92: 261-265.
- Balcerak RJ, Sisto JM, Bosack RC (1988) Cervicofacial necrotizing fasciitis: Report of three cases and literature review. J Oral Maxillofac Surg 46: 450-459.
- Banerjee AR, Murty GE, Moir AA (1996) Cervical necrotizing fasciitis: A distinct clinicopathological entity? J Laryngol Otol 110: 81-86.
- Jain D, Kumar Y, Vasishta RK, Rajesh L, Pattari SK, et al. (2006) Zygomatic Necrotizing Fascitis in immunocompetent patients: A series of 18 cases: Mod Pathol 19: 1221-1226.
- Giuliano A, Lewis F Jr, Hadley K, Blaisdell FW (1977) Bacteriology of necrotizing fasciitis. Am J Surg 134: 52-57.
- Schlievert PM, Assimacopoulos AP, ClearyPP (1996) Severe Invasive group A streptococcal disease: Clinical description and mechanisms of pathogenesis. J Lab Clin Med 127: 13-22.
- Enright MC, Spratt BG, Kalia A, Cross JH, Bessen DE (2001) Multilocus sequence typing of Streptococcus pyogenes and the relationships between emm type and clone. Infect Immunol 69: 2416-2427.
- Freischlag JA, Ajalat G, Busuttil RW (1985) Treatment of necrotizing soft tissue infections. The need for a new approach. Am J Surg 149: 751-755.
- Brook I, Frazier EH (1995) Clinical and microbiological features of necrotizing fasciitis. J Clin Microbiol 33: 2382-2387.
- Engleberg NC, Heath A, Miller A, Rivera C, DiRita VJ (2001) Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J Infect Dis 183: 1043-1054.
- Jain S, Nagpure PS, Singh R, Garg D (2008) Minor trauma triggering cervicofacial necrotizing fasciitis from odontogenic abscess. J Emerg Trauma Shock 1: 114-118.
- Stoykewych AA, Beecroft WA, Cogan AG (1992) Fatal necrotizing fasciitis of dental origin. J Can Dent Assoc 58: 59-62.
- Fliss DM, Tovi F, Zirkin HJ (1990) Necrotizing soft-tissue infections of dental origin J Oral Maxillofac Surg: 48: 1104-1108.
- De Backer T, Bossuyt M, Schoenaers J (1997) Management of necrotizing fasciitis in the neck. J Craniomaxillofac Surg 24: 366-371.
- Brun-Buisson CJ, Saada M, Trunet P, Rapin M, Roujeau JC, et al. (1985) Haemolytic streptococcal gangrene and non-steroidal anti-inflammatory drugs. Br Med J 290:1786.
- Whitesides L, Cotto-Cumba C, Myers RA (2000) Cervical necrotizing fasciitis of odontogenic origin: A case report and review of 12 cases. J Oral Maxillofac Surg 58: 144-151.
- Dellinger EP (1981) Severe necrotizing soft-tissue infections. Multiple disease entities requiring a common approach. JAMA 246: 1717-1721.
- Marioni G, Bottin R, Tregnaghi A, Boninsegna M, Staffieri A (2003) Craniocervical necrotizing fasciitis secondary to parotid gland abscess. Acta Otolaryngol 123: 737-740.
- Shupak A (1991) Letter to editor. Ann Otol Rhinol Laryngol 100: 132.
- Umeda M, Minamikawa T, Komatsubara H, Shibuya Y, Yokoo S, et al. (2003) Necrotizing fasciitis caused by dental infection: A retrospective analysis of 9 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 95: 283-290.
- Dale RA, Hoffman DS, Crichton RO, Johnson SB (1999) Necrotizing fasciitis of the head and neck: Review of the literature and report of a case. Spec Care Dentist 19: 267-274.
- Raboso E, Llavero MT, Rosell A, Martinez-Vidal A (1998) Craniofacial necrotizing fasciitis secondary to sinusitis. J Laryngol Otol 112: 371-372.
- Skitarelic N, Mladina R, Matulic Z, Kovacic M (1999) Necrotizing fasciitis after peritonsillar abscess in an immunocompetent patient. J Laryngol Otol 113: 759-761.
- Hadfield PJ, Motamed M, Glover GW (1996) Synergistic necrotizing cellulitis resulting from peri-tonsillar abscess. J Laryngolol Otol 110: 887-890.
- Feinerman IL, Tan HK, Roberson DW, Malley R, Kenna MA (1999) Necrotizing fasciitis of the pharynx following adenotonsillectomy. Int J Pediatr Otorhinolaryngol 48: 1-7.
- Walshaw CF, Deans H (1996) CT findings in necrotizing fasciitis--A report of four cases. Clin Radiol 51: 429-432.
- Kaplan DM, Fliss DM, Shulman H, Leiberman A (1995) Computed tomographic detection of necrotizing soft tissue infection of dental origin. Ann Otol Laryngol 104: 164-166.
- Saiag P, Le Breton C, Pavlovic M, Fouchard N, Delzant G, et al. (1994) Magnetic resonance imaging in adults presenting with severe acute infectious cellulitis. Arch Dermatol 130: 1150-1158.
- Strauss MB, Chung MM, Hart GB, Weinstein P (1996) The role of hyperbaric oxygenation therapy for Necrotizing Fasciitis. World J Med 164: 363-364.
|
| |
| |