| Rapid Communication |
Open Access |
|
| Bioavailability of Long Acting Tramadol in Mexican Healthy Volunteers |
| López-Gamboa1*, Canales-Gómez2, Castro-Sandoval1, Núñez Tovar E1, Campos-Lara1, Melchor-Baltazar1 and Palma-Aguirre1 |
| 1Centro de Estudios CientÃficos y ClÃnicos Pharma, S.A. de C.V., Mexico City, Mexico |
| 2Productos Medix S.A. de C.V., México |
| *Corresponding author: |
Mireya López-Gamboa
Centro de Estudios CientÃficos y ClÃnicos Pharma
S.A. de.C.V., Amores #320; Col. Del Valle
Del. Benito Juárez, C.P. 03100
D. F. México City, México
E-mail: dralopezg@gmail.com; mlopez@cecycpharma.com |
|
| Â |
| Received June 14, 2012; Published October 30, 2012 |
| Â |
| Citation:Gamboa L, Gómez C, Sandoval C, Núñez Tovar E, Lara C, et al. (2012) Bioavailability of Long Acting Tramadol in Mexican Healthy Volunteers. 1:425. doi:10.4172/scientificreports.425 |
| Â |
| Copyright: © 2012 Gamboa L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| Tramadol hydrochloride is a synthetic, centrally acting analgesic, used for the relief of moderate to severe acute and chronic pain. Immediate-release tramadol have a short duration of therapeutic effect and are generally less effective for pain control than other opioid derivatives. The aim of this study was to investigate the pharmacokinetic profile of long release formulation of tramadol. Twelve healthy volunteers were included into the study. After 12-hour (overnight) fast, subjects received a single capsule of long release of tramadol 100 mg dose. For the analysis of pharmacokinetic properties, blood samples were drawn at baseline, 0.5, 1.0, 1.25, 1.5, 1.75, 2.0, 2.5, 3.0, 4.0, 6.0, 8.0, 12.0 and 24.0 hours after dosing. The tramadol pharmacokinetic values were Cmax of 245.60 ± 54.26 ng/mL, tmax of 3.79 ± 0.50 h, AUC0-t of 2,621.39 ± 780.21 hr.ng/mL, AUC0-∞ of 2,917.16 ± 980.29 hr.ng/mL, t1/2 of 6.24 ± 1.12 h, Cl of 38.18 ± 13.14 L/h and Vd of 328.71 ± 74.44 L. The pharmacokinetic (PK) values of long acting tramadol of our study were consistent with PK results of other populations that used the extended release formulation of tramadol. |
| Â |
| Keywords |
| Â |
| Tramadol; Pharmacokinetics; Human; HPLC; MS-MS; Long release capsules |
| Â |
| Introduction |
| Â |
| Tramadol hydrochloride is a synthetic, centrally acting analgesic, which has been used since 1977 for the relief of moderate to severe acute and chronic pain. Tramadol is rapidly and almost completely absorbed after an oral administration. However, its mean absolute bioavailability is only 65–70% due to the first-pass hepatic metabolism [1,2]. The extent of bioavailability increases to 77% after the rectal administration of tramadol suppositories [3] and to 100% after an intramuscular administration [4]. The peak plasma concentration is reached in 0.17–1.5 h following the intramuscular administration [5], in 0.5–1.7 h after the oral administration of drops [2], in 1–3 h after the oral administration of capsules [1] and in 2–6 h after the rectal administration of suppositories [3].The biotransformation of tramadol has been investigated in diverse kind of animals and humans [5]. The principal metabolic pathways, O-and N-desmethylation, involve cytochrome P-450 isoenzymes 2D6 (pivotal), 2B6 and 3A4 [6]. Approximately 10–30% of the parent drug is excreted unmetabolised in the urine [7] Tramadol plasma protein binding is shown to be 4–20% [6]. The mean terminal half-lives of tramadol and O-desmethyltramadol are about 5–7 hours [1-6]. Immediate-release tramadol have a short duration of therapeutic effect and are generally less effective for pain control than other opioid derivatives. Common criticisms of tramadol also include the patient's tendency to develop dependence on the drug itself. The newer extended-release tramadol formulation was designed to increase efficacy, duration of therapeutic effect, tolerance, compliance, and facilitate discontinuation. The aim of this study was to investigate the pharmacokinetic profile of long release formulation of tramadol 100 mg in Mexicans with special reference in comparing these PK parameters with other populations. |
| Â |
| Methods |
| Â |
| Twelve healthy volunteers (six females), aged between 18 and 55 years were included based on their medical history, clinical examination results, and routine laboratory tests. Subjects with HIV or hepatitis B or C virus were excluded. All healthy volunteers were selected based on information from a database of the Centro de Estudios CientÃficos y ClÃnicos Pharma S. A. de C. V. (CECYC Pharma Clinical Unit), Mexico City, Mexico. All eligible subjects provided written informed consent for participation in the study. Subjects were compensated for participation. |
| Â |
| Subjects |
| Â |
| Twelve healthy volunteers (six females), aged between 18 and 55 years were included based on their medical history, clinical examination results, and routine laboratory tests. Subjects with HIV or hepatitis B or C virus were excluded. All healthy volunteers were selected based on information from a database of the Centro de Estudios CientÃficos y ClÃnicos Pharma S. A. de C. V. (CECYC Pharma Clinical Unit), Mexico City, Mexico. All eligible subjects provided written informed consent for participation in the study. Subjects were compensated for participation. |
| Â |
| Study design |
| Â |
| The study was approved by the ethics and research committees of the Centro de Estudios CientÃficos y ClÃnicos Pharma S. A. de C. V. The study was carried out in accordance with the principles of the Declaration of Helsinki and its amendments and the International Conference of Harmonisation Guidelines for Good Clinical Practice. A single, open-label design was used. The subjects arrived at the clinical Unit the day before the beginning of the study. After that, subjects were fasted for 12 hours overnight before drug administration. Blood was drawn for baseline plasma measurements in the following manner: a 20-G catheter (Jelco®, Plus, Medex Medical Ltd. Ascot, United Kingdom) was placed in a suitable forearm vein and a 6-mL blood sample was drawn into a heparinized vacuum tube (Vacutainer®, Becton-dickinson and Company, Franklin Lakes, New Jersey). Subjects received a single long release capsule of tramadol 100 mg (Prontofort®), manufactured by Productos Medix, S. A. de C. V, given with 250 mL of water, and additional blood samples were drawn at 0.5, 1.0, 1.25, 1.5, 1.75, 2.0, 2.5, 3.0, 4.0, 6.0, 8.0, 12.0, 24.0 hours after study drug administration. Plasma levels obtained bay centrifugation (2000 g for 10 minutes at room temperature) and stored frozen at -70°C until analyzed using high-performance liquid chromatography (HPLC) with a MS-MS detector. |
| Â |
| Determination of plasma tramadol concentrations |
| Â |
| Tramadol plasma levels were determined using HPLC with MS_MS detector as adapted by personnel of CECYC Pharma Analytical Unit, in which venlafaxine was used as internal standard, based on the methods of Nobilis and colleagues [4]. Briefly, 0.2 mL of plasma and 0.1 mL of internal standard (venlafaxine), were mixed by shaking in a test tube for 1 minute. The supernatant was filtered through a regenerated cellulose syringe filter (pore size, 0.45 micron) and injected into the chromatographic system. The chromatographic conditions were: column (Zorbax XDB C18, 2.1×50 mm, 5 μm), ammonium formiate solution at 0.025%: Acetonitrile (60:40 v/v) used as mobile phase. The injection volume was 5 μL, the flow rate was maintained constant at 0.3 mL/min, the column temperature was of 30°C and the retention times were between 0.4-0.8 minutes for tramadol and between 0.4-0.8 minutes for venlafaxine. The concentration ranges were from 5.0 a 300.0 ng/mL. The extraction method consisted in a liquid-liquid extraction, with a mixure of methyl-terbutil-ether. The MS-MS detector conditions were source temperature of 120°C, desolvation temperature of 400°C, multiplier of 650.0 V and Capilar of 3 kV. |
| Â |
| Tolerability |
| Â |
| Tolerability was assessed by monitoring vital signs (blood pressure, heart rate, and body temperature) at baseline, 4.5, 11.5 and 23.5 hours, and subject interviews regarding the potential of adverse events (AEs) during the whole study. |
| Â |
| Pharmacokinetics and statistical analysis |
| Â |
| Individual plasma concentration-time curves were constructed; Cmax and tmax were directly obtained from the curve. AUC from time 0 (baseline) to 24 hours (AUC0-24) was calculated using the trapezoidal rule. From the terminal log-decay phase, elimination rate constant (ke) was estimated using linear regression, and t1/2 was estimated using the following equation: |
| Â |
| t1/2 = ln2/ke |
| Â |
| Where ln was defined as the natural log; and extrapolation of AUC from baseline to infinity (AUC0-∞) was calculated as follows: |
| Â |
| AUC0-∞ = AUC0-24 + (C24/ke) |
| Â |
| Where, C72 was defined as concentration at 24 hours. |
| Â |
| Results |
| Â |
| A total of 12 subjects were enrolled in the study. Subjects had the following characteristics: age range 18 to 49 years (mean ± SD of 23.2 ± 2.9 years), mean weight of 63.8 ± 7.8 kg, and mean height of 1.65 ± 0.08 m (Table 1). |
| Â |
|
|
Table 1: Descriptive statistics of demographic characteristics of the volunteers (n=12). |
|
| Â |
| Pharmacokinetic parameters |
| Â |
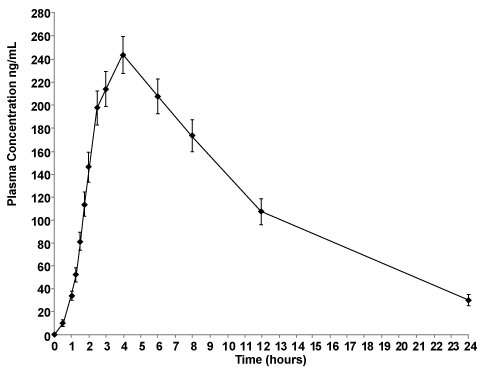
| Individual values of Cmax, tmax, AUC0-t, AUC0-∞, t½, Cl (clearance) and Vd (apparent volume of distribution) are shown in table 2. Pharmacokinetic mean and SD values were Cmax of 245.60 ± 54.26 ng/mL, tmax of 3.79 ± 0.50 h, AUC0-t of 2,621.39 ± 780.21 hr.ng/mL, AUC0-∞ of 2,917.16 ± 980.29 hr.ng/mL, t1/2 of 6.24 ± 1.12 h, Cl of 38.18 ± 13.14 L/h and Vd of 328.71 ± 74.44 L (Table 3). Figure 1 shows the mean (SD) plasma tramadol concentrations after drug administration. |
| |
| Â |
|
|
Table 2: Individual tramadol pharmacokinetic parameters after 100 mg administration in Mexican healthy volunteers. |
|
| Â |
|
|
Table 3: Descriptive statistics of tramadol 100 mg long acting capsules PK parameters. |
|
| Â |
|
|
Figure 1: Main plasma profile of tramadol 100 mg long acting capsules. |
|
| Â |
| Safety |
| Â |
| Some adverse effects occurred in the volunteers during the study after tramadol administration of a 100 mg long release capsules as follows: three volunteers had mild dizziness, and occurred into 4 hours post-dose and disappeared spontaneously without drug medication. These adverse events were classified with probable association with the study drug. Two volunteers had nausea of mild intensity and vomits in between 7 and 10 hours post-dosing. These adverse events were classified with probable association with the study drug and they were controlled with ranitidine 50 mg/10 mL, methoclopramide 10 mg/2 mL and a saline solution 1000 mL by intravenous route. |
| Â |
| Discussion |
| Â |
| Lintz et al. [9] determined the absolute bioavailability of tramadol hydrochloride 200 mg in healthy volunteers by oral and intravenous routes. The absolute bioavailability of tramadol in capsules was 68 ± 13% with a range of 41-84%. Peak serum concentrations of 280 ± 49 ng/ml were reached 2 hours after oral administration of two capsules of tramadol. The terminal phase of the biological half-lives of tramadol was 5.1 ± 0.8 h (oral) and 5.2 ± 0.8 h (intravenous). Karhu conducted a three-way crossover study in healthy volunteers in order to characterize the pharmacokinetics and to assess the dose proportionality of 100 mg, 200 mg and 300 mg strengths of a novel once-a-day tramadol controlled-release tablet. PK results were similar with respect the PK data from Mexican volunteers of our study. |
| Â |
| Hernandez-Lopez and colleagues compared the pharmacokinetic profile and oral bioavailability of two tramadol 200 mg tablets with following single-dose administration in 26 healthy volunteers from Spain. Results also were similar than Mexican results. Racial and ethnic differences in drug-metabolizing ability associated with genetic or cultural/environmental factors are not only well recognized but also important in understanding interindividual variability in drug responsiveness. Subrahmanyam demonstrated that cis-tramadol can be metabolized to tramadol metabolites by CYP2B6 and CYP3A4 isoforms. There are information about the pharmacokinetics of drugs metabolized by the CYP 3A4 in Mexican population, where there is interethnic evidence of differences in the PK results of nifedipine [9] midazolam [10], cyclosporine [11] and sildenafil [12]. Although Poland and colleagues [13] reported that CYP 3A activities do not differ between Mexicans [14] and European Americans [15]. In the case of tramadol, we also did not see ethnic differences with respect to Caucasian volunteers [16]. |
| Â |
| Further clinical studies with the long acting formulation had to be done in patients in order to evaluate the pharmacodynamic activity of the tramadol formulation used in our study. |
| Â |
| Acknowledgements |
| Â |
| The study was supported entirely by Laboratories Productos Medix S. A. de C. V., Mexico City, Mexico. |
| Â |
| |
| References |
| Â |
- Castañeda-Hernández G, Palma-Aguirre JA, Montoya-Cabrera MA, Flores-Murrieta FJ (1996) Interethnic variability in nifedipine disposition: reduced systemic plasma clearance in Mexican subjects. Br J Clin Pharmacol 41: 433-434
- Castañeda-Hernández G, Hoyo-Vadillo C, Palma-Aguirre JA, Flores-Murrieta FJ (1993) Pharmacokinetics of oral nifedipine in different populations. J Clin Pharmacol 33: 140-5
- Scott LJ, Perry CM (2000) Tramadol: A Review of its Use in Perioperative Pain. Drugs 60: 139-176.
- Chavez-Teyes L, Castañeda-Hernández G, Flores-Murrieta FJ (1999) Pharmacokinetics of midazolam in Mexicans: evidence of interethnic variability. Clin Drug Invest 17: 233-239.
- Flores-Murrieta FJ, Castañeda-Hernández G, Granados-Soto V, Herrera JE (2000) Increased bioavailability of sildenafil in Mexican men. JAMA 283: 1825-1826.
- Hernandez-Lopez C, Martinez-Farnos L, Karhu D, Perez-Campos T, Rovira S, et al. (2006) Comparative bioavailability between two Tramadol once-daily oral formulations. Methods Find Exp Clin Pharmacol 28: 373-378.
- Lee CR, McTavish D, Sorkin EM (1993) Tramadol. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in acute and chronic pain states. Drugs 46: 313-340
- Lintz W, Barth H, Osterloh G, Schmidt-Böthelt E (1986) Bioavailability of enteral tramadol formulations. 1st communication: capsules. Arzneimittelforschung 36: 1278-83.
- Lintz W, Barth H, Becker R, Frankus E, Schmidt-Böthelt E (1998) Pharmacokinetics of tramadol and bioavailability of enteral tramadol formulations. 2nd communication: drops with ethanol. Arzneimittelforschung 48: 436-445.
- Lintz W, Barth H, Osterloh G, Schmidt-Böthelt E (1998) Pharmacokinetics of tramadol and bioavailability of enteral tramadol formulations. 3rd Communication: suppositories. Arzneimittelforschung. 48: 889-899.
- Karhu D, El-Jammal A, Dupain T, Gaulin D, Bouchard S (2007) Pharmacokinetics and dose proportionality of three Tramadol Contramid OAD tablet strengths. Biopharm Drug Dispos 28: 323-30.
- Lee CR, McTavish D, Sorkin EM (1993) Tramadol. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in acute and chronic pain states. Drugs. 46: 313-40.
- Nobilis M, Kopecký J, Kvetina J, Chládek J, Svoboda Z, et al. (2002) High-performance liquid chromatographic determination of tramadol and its O-desmethylated metabolite in blood plasma. Application to a bioequivalence study in humans. J Chromatogr A 949: 11-22.
- Palma-Aguirre JA, González-Llaven J, Flores-Murrieta FJ, Castañeda-Hernández G (1997) Bioavailability of oral cyclosporine in healthy Mexican volunteers: evidence for interethnic variability. J Clin Pharmacol 37: 630-634.
- Poland RA, Lin KM, Nuccio C, Wilkinson GR (2002) Cytochrome P450 2E1 and 3A activities do not differ between Mexicans and European Americans. Clin Pharmacol Ther 72: 288-93.
- Subrahmanyam V, Renwick AB, Walters DG, Young PJ, Price RJ, et al. (2001) Identification of cytochrome P-450 isoforms responsible for cis-tramadol metabolism in human liver microsomes. Drug Metab Dispos 29: 1146-55
|
| Â |
| Â |