| Research Article |
Open Access |
|
| Ch Paramageetham1 and G Prasada Babu2* |
| 1Department of Microbiology, Sri Venkateswara University, Tirupati, Andhra Pradesh, India |
| 2Department of Botany, Sri Venkateswara University, Tirupati, Andhra Pradesh, India |
| *Corresponding author: |
G Prasada Babu
Department of Botany
Sri Venkateswara University
Tirupati- 517502, Andhra Pradesh, India
E-mail: prag.babu@gmail.com |
|
| Â |
| Received October 13, 2012; Published October 26, 2012 |
| Â |
| Citation: Paramageetham Ch, Prasada Babu G (2012) Antagonistic Activity of Fluorescent Pseudomonads against a Polyphagous Soil Born Plant Pathogen – Sclerotium Rolfsii. 1:436. doi:10.4172/scientificreports.436 |
| Â |
| Copyright: © 2012 Paramageetham Ch, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| A total of 35 fluorescent Pseudomonad strains were isolated from forest litter of seshachalam hill range. Out of 35 bacterial isolates 19 were found to be antagonistic to Sclerotium rolfsii in in vitro conditions. The growth inhibition zone of Sclerotium by fluorescent Pseudomonads on potato dextrose agar varied from 4-9 mm with an average of 6.2 mm. Among the isolated fluorescent Pseudomonads PSTPT13 found to be most potential. All the 19 isolates were characterized morphologically, biochemically and functionally. The mechanism of fungal toxicity was also observed by the production of HCN, siderophore and cell wall degrading enzymes. In this study we observed fungistatic activity is mainly due to the production of HCN, Siderophore and protease enzyme. This is the first report on fluorescent Pseudomonads isolated from forest litter of Seshachalam hill range the first ever biodiversity reserve of India with antagonistic activity against Sclerotium. |
| Â |
| Keywords |
| Â |
| Polyphagous; Antagonism; Fluorescent pseudomonads; Sclerotium rolfsii |
| Â |
| Introduction |
| Â |
| Sclerotium rolfsii is a polyphagous soil borne pathogen infecting over 500 plant species worldwide causing huge losses. Though the fungus is seed and soil borne; soil borne inoculum is more important in causing infection and disease development. For the soil borne pathogens, use of fungicides is not practical due to exorbitant cost and environmental hazards involved. Hence integrated management of the disease using biocontrol agents and chemicals is the best. The pathogen is distributed in tropical and subtropical regions of the world where high temperature prevails. The fungus has a wide host range of 500 species in about 100 families including vegetables, flowers, cereals, forage plants and weeds. Some of the common hosts include Legumes, Crusifers, Tomato, Chrysanthemum, Peanuts and Tobacco in which the pathogen causes a great economic loss. In ground nut, it caused 25% of seedling mortality in the cultivar JL- 24 at parbhani [1]. Thiribhuvanamal et al. [2] observed that 30% of crop loss in tomato was due to S. rolfsii. Harinath Naidu [3] reported that S. rolfsii caused 40.05% mortality in Crossandra in Chittoor district of Andhra Pradesh. |
| Â |
| Several chemical pesticides are used to manage this disease [4-6]. Indiscriminate use of chemical pesticides in modern agriculture has resulted in the development of several problems such as pesticide resistance in pest resurgence of target and non-target pests, destruction of beneficial organisms like honey bees and chemical residues in food, feed and fodder. More over fungicidal application as seed or soil treatment however has been found to be ineffective against these pathogens as the propagules are capriciously distributed in the soil and often beyond the reach of chemicals [7]. Biological control therefore holds a promise as a strategy for disease management and it is environment friendly too. Antagonistic bacteria especially fluorescent pseudomonads have been widely used against a number of phytopathogens [8,9]. For successful functioning of introduced microbial bio-inoculants and their influence on soil, health efforts have been made to explore soil microbial diversity of indigenous community their distribution and behavior in soil habitats [10]. Seshachalam hills that forms part of the Eastern Ghats which spreads in parts of Chittor and Kadapa districts in Indian state of Andhra Pradesh. The hill range has been declared as first ever biodiversity reserve in Andhra Pradesh by Union Ministry of Environment and forests recently in 2010. Keeping this in view and the growing importance of biological control agents, the present study was carried out. The main objective was to evaluate the biocontrol efficiency of indigenous fluorescent psuedomonads against Sclerotium rolfsii. There appears to be no report on the antagonistic effect of fluorescent pseudomonads isolates from seshachalam hills of Chittor district. |
| Â |
| Materials and Methods |
| Â |
| Collection of soil samples |
| Â |
| Soil samples were collected from forest litter of Seshachalam hills, Andhra Pradesh. Randomized block design was employed to collect the samples. Collected soils were sealed in sterile polyethylene bags. |
| Â |
| Isolation of bacteria |
| Â |
| One gram soil from different sampling sites was placed in 9 ml of saline solution and incubated for 2 hours in an orbital shaking incubator at 180 rpm. Later a loop of the resulting bacterial suspension was spread plated on King’s B Agar medium [11] and incubated at 37°C. After 2 days the colonies were screened for fluorescence under UV light (366 nm). |
| Â |
| Selection of antagonistic bacteria |
| Â |
| A total of 35 bacterial isolates obtained from litter were screened for antagonism against Sclerotium rolfsii. Antagonism of bacteria against Sclerotium was examined using a modified method of Montealerge et al. [12]. A loopful of culture from each purified bacterial isolate was inoculated into 50 ml of King’s B agar broth and incubated for 48 hours at 37°C. Subsequently, 100 μl of bacterial suspension of each isolate was placed on different 10 mm diameter sterile paper discs (Whatman, UK) four different discs were spaced around a central 10 mm plug of 2 day old Sclerotium on potato dextrose agar. The plate was incubated for 7 days at 30°C and the size of the inhibition zone of hyphal growth was determined. Bacteria which showed no suppression of fungal growth were discussed. The inhibition test was replicated three times. The active bacterial isolates were preserved in 20% glycerol at -20°C. |
| Â |
| 19 bacterial isolates showed antagonistic activity in the pre evaluation test were subjected to further confirmation by the standard co-inoculation PDA technique [13]. The bacterial plugs (6 mm diameter) were removed from a 48 hrs grow culture on king’s B plate. The bacterial plugs were transferred to the surface of PDA plates, which had been inoculated with fungal spore suspension (or) mycelial plug. After the plates were incubated at 28°C for 3 days radial growth percentage of the test fungi was measured using following formula: |
| Â |
| RI(%)= Rc-Ri/Rc *100 |
| Â |
| where, |
| Â |
| RI=Radial growth inhibition |
| Â |
| Rc= Radial growth in control plates |
| Â |
| Ri= Radial growth in incubated plates |
| Â |
| Phenotypic and biochemical characteristics of antagonistic bacteria |
| Â |
| Phenotypic characters like grams reaction, levan production, optimum growth temperature, fluorescence, Gelatin hydrolysis, citrate utilization test, Oxidase, β-galactosidase activity, Catalase test, Indole production were conducted for 19 bacterial isolates which showed antagonistic activity. |
| Â |
| Assimilation of carbohydrates |
| Â |
| Substrate utilization profiles were tested using Hi carbohydrates (Himedia, Mumbai, India) Cell suspension was established in sterile saline using 24 hours grown culture. The density of the suspension was made to 0.5 O.D at 620 nm. An aliquot of 50 μl of this suspension was inoculated at 30°C for 48 hrs and on to the substrates such as lactose, xylose, fructose, Galactose, glycerol, Trehalose, Mannitol, Sucrose, Ribose, Glucose and incubated at 30°C for 48 hrs. |
| Â |
| Cell wall degrading enzymes |
| Â |
| Cell wall degrading enzymes like protease, cellulase and pectinase were also studied [13,14]. |
| Â |
| Plant growth promoting traits |
| Â |
| Phosphate solubilizing enzyme production was assessed by using pikovskaya medium [15]. For all the isolates siderophore production was also observed. |
| Â |
| Volatile toxicity of antagonistic fluorescent Pseudomonads |
| Â |
| In order to identify volatile toxicity in antagonistic strains HCN production test was conducted by using filter paper pre soaked in picric acid solution. The productions of volatile toxic compounds were assured by taking the following criteria. |
| Â |
|
No colour change
|
-
|
No HCN production
|
-
|
Â
|
|
Brownish coloration
|
-
|
Weak HCN Production
|
-
|
 Weak HCN production
|
|
Brownish to Orange
|
-
|
Moderate HCN production
|
-
|
 Moderate HCN production
|
|
Complete Orange
|
-
|
Strong HCN production
|
-
|
 Strong HCN production
|
|
| Â |
| Numerical taxonomy |
| Â |
| A binary code matrix of each strain was constructed linearly composing presence (1) /absence (0) of data derived from the biochemical tests of antagonistic fluorescent Pseudomonads. SPSS.16 version was used to compute similarities or dissimilarities in the form of an average taxonomic distance which was used to perform hierarchical, agglomerative and neighbor joining clustering. Dendrogram was constructed from the similarity matrix obtained in the SPSS.16 program. |
| Â |
| Results |
| Â |
| A total of 35 strains of fluorescent Pseudomonads were isolated from forest litter. Most of the isolated bacteria developed pale green to dark green pigmentation on king’s B agar and released a sweet grape like odour and pyocyanine pigment. This was an indication that isolated bacteria were pseudomonads. By exposing the plates to UV light fluorescent pseudomonads were picked up. |
| Â |
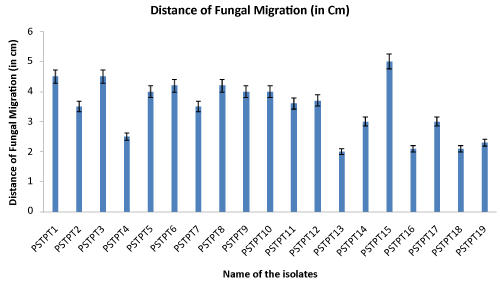
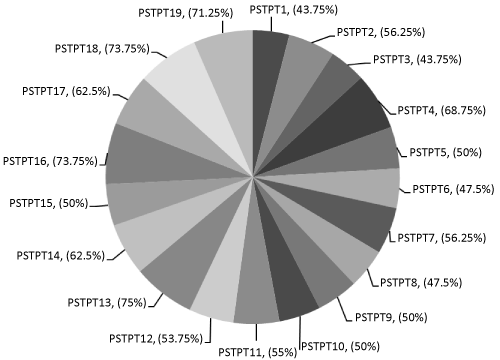
| Among them a total of 19 isolates were found to inhibit mycelial growth on PDA plates in a triplicate assay. All antagonistic isolates produced an inhibition zone varied from 2.0 cm to 4.5 cm (Figure 1) and radial growth inhibition percentage from 43.75% to 73.75%.However isolates PSTPT16 and PSTPT18, were found to be potential antagonists against Sclerotium with almost 73.75% of radial growth inhibition (Figure 2) percentage. |
| Â |
|
|
Figure 1: Sclerocium mycelial migration by antagonistic fluorescent Pseudomonads. |
|
| Â |
|
|
Figure 2: Radial growth inhibition percentage against Sclerotium rolfsii by antagonistic fluorescent Pseudomonads. |
|
| Â |
| Phenotypic Characterization of fluorescent Pseudomonads: All bacteria showed positive results for fluorescence, oxidase and catalase. A total of 11 isolates were positive for levan production and 8 isolates were negative .Most of the isolates were able to grow at optimum temperature from 25°C-30°C. However isolates PSTPT 15, 16 and 17 were grown at 40°C. Only two isolates showed optimum temperature at 0oC. About 57% of the isolates were positive for gelatin liquefaction and 43% were negative. All the isolates were able to utilize citrate except isolate PSTPT 16. Further Isolate17 was the only positive for ONPG production. All strains are able to utilize glucose but exhibited varying degrees of utilization towards other carbon sources such as lactose, mannose, galactose, ribose etc. (Table 1). |
| Â |
|
|
Table 1: Phenotypic and Biochemical characteristics of antagonistic fluorescent Pseudomonads. |
|
| Â |
| Production of Cell wall degrading enzymes |
| Â |
| The functional characterization demonstrated the diversity and fungitoxic ability of antagonistic fluorescent Pseudomonads. These strains did not produce cellulase. But all the strains are able to produce pectin and protease (Table 2). |
| |
| Â |
|
|
Table 2: Production of cell wall degrading enzymes from antagonistic Fluorescent Pseudomonads. |
|
| Â |
| Mechanism of biological control |
| Â |
| In vitro HCN by the antagonistic strains was tested by the picric acid assay method and 78.9% isolates were found to produce HCN. However isolate PSTPT 5, 6 and 19 were strong HCN producers which turned the colour of the filter paper in to complete orange. Three isolates were moderate HCN producer and 7 were weak producer of HCN (Table 3). |
| Â |
|
|
Table 3: Volatile toxicity of antagonistic Fluorescent Pseudomonads. |
|
| Â |
| Numerical analysis |
| Â |
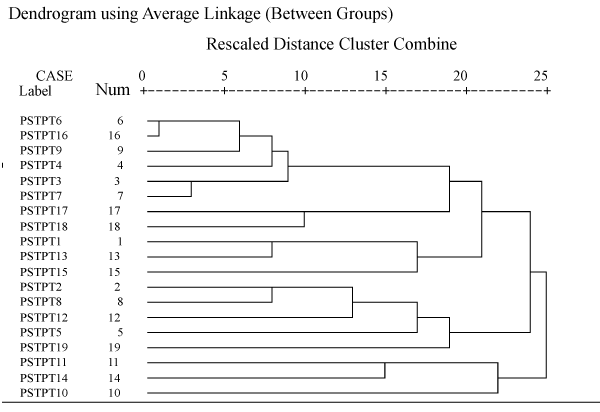
| Numerical analysis of phenotypic characteristics revealed a high degree of polymorphism. All the strains were grouped in to two different phenons (Figure 2). The similarity range among antagonistic strains was 0.37-0.94 (Table 4). The first phenon consists of six strains (PSTPT 6, 16, 9, 4, 3, 7) and second phenon consists of a total of 13 strains (Figure 3). |
| Â |
|
|
Figure 3: Dendogram using Average Linkage (Between Groups). |
|
| Â |
|
|
Table 4: Similarity matrix for biochemical characteristics of antagonistic fluorescent pseudomonads. |
|
| Â |
| Production of plant growth promoting traits |
| Â |
| All the strains were able to produce plant growth promoting traits like phosphatase and siderophore (Table 5). |
| Â |
|
|
Table 5: Growth promoting traits of antagonistic fluorescent pseudomonads. |
|
| Â |
| Discussion |
| Â |
| Soil is considered as a store house of microbial activity. These functions of soil microorganisms are central to the decomposition process and nutrient cycling. They play an important role in soil processes that determine plant productivity. The hill chain of Eastern Ghats is recently recognized as Biodiversity reserve. In spite of this there is no report on the diversity of fluorescent Pseudomonads in this region. Among the 35 isolates 19 isolates exhibited antagonistic activity against Sclerotium rolfsii. The biological control of soil borne pathogens with antagonistic bacteria particularly Pseudomonas sp., belonging to plant growth promoting Rhizobacteria has received prominent attention because of the dual role of these bacteria in plant growth promotion and diseases control [16]. Fluoroscent pseudomonad strains found to be effective against Sclerotium rolfsii [17]. Cook [9] reported that certain plant associated bacteria particularly fluorescent pseudomonads have been exploited for suppression of crop diseases. Similar results on the effectiveness of fluorescent pseudomonads against plant pathogenic fungi like Fusarium, Rhizoctonia, Sclerotium, Pythium [18-24] and bacteria like Ralstonia solanacearum and Xanthomonas campestris have been reported earlier .The effectiveness of fluorescent pseudomonads against multiple pathogens are also known. |
| Â |
| Our work demonstrates the ability of fluorescent pseudomonads to produce fungistatic metabolites such as siderophores, HCN and protease by the bacteria. Pseudomonas sp. are known to produce volatile compounds. One such metabolite is HCN. Afsharmanesh et al. [23] suggested that fungal growth is mainly inhibited by HCN production and siderophore production. Apart from the biocontrol potential, fluorescent pseudomonads possess other functional properties like, mineral phosphate solubilisation, production of plant growth promoting substances and enzyme activity. Besides testing the fluorescent pseudomonads for beneficial functions like Phosphate solubilisation, PGPS production and biocontrol potential, their ability to produce commercially important enzymes like protease and chitinase was also examined. Out of the 19 antagonistic isolates, all the isolates are able to produce protease but none of the isolates produced Chitinase and cellulase. The results of present investigation indicated a high degree of functional diversity among antagonistic fluorescent pseudomonads isolated from forest litter of Eastern Ghats. |
| Â |
| Conclusion |
| Â |
| Strains reported in this study suppress Sclerotium effectively by single or multiple modes of action. Results also revealed that the antifungal activities and other plant beneficial traits appear to be the general and genetically dispersed traits of fluorescent pseudomonads. Knowledge on phenotypic and functional traits of antagonistic bacteria will help to determine their fitness for successful bio-fertilization and biological control. This study reveals for the first time the presence of bacteria with antagonistic activity against Sclerotium rolfsii in Eastern Ghats forest litter an untapped resource. It also provides essential information to develop broad spectrum biocontrol agent. |
| Â |
| |
| References |
| Â |
- Montealegre JR, Reyes R, Perez LM, Herrera R, Silva P, et al. (2003) Selection of bioantagonistic bacteria to be used in biological control of Rhizoctonia solani in Tomato. Electronic Journal of Biotechnology 6: 115-127.
- Chernin L, Ismailov Z, Haran S, Chet I (1995) Chitinolytic Enterobacter agglomerans Antagonistic to Fungal Plant Pathogens. Appl Environ Microbiol 61: 1720-1726.
- Cahelan AJ, Hartel PG, Fuhrmann JJ (1999) Screening for plant growth promoting rhizobacteria to promote early soybean growth. Soil science Society of America Journal 63: 1670-1680.
- Castric KF, Castric PA (1983) Method for rapid detection of cyanogenic bacteria. Appl Environ Microbiol 45: 701-702.
- Leon M, Yaryura PM, Montecchia MS, Hernandez AI, Correa OS, et al. (2009) Antifungal activity of selected Indigenous Pseudomonads and Bacillus from the soybean Rhizosphere. Int J Microbiol.
- Rokni Zadeh H, Khavazi K, Asgharzadeh A, Hosseini-Mazinani M, De Mot R (2008) Biocontrol of Pseudomonas sevastanoi, causative agent of olive knot disease: antagonistic potential of non – pathogenic rhizosphere isolates of fluorescent Pseudomonads. Commun Agric Appl Biol Sci 73: 199-203.
- King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44: 301-307.
- Asha BB, Chandra Nayaka S, UdayaShankar AC, Srinivas C, Niranjana SR (2011) Biological control of F. oxysporium f.sp. Lycopersici causing wilt of tomato by Pseudomonas fluorescens. Int J Microbiol Res 3: 79-84.
- Cook RJ (1993) Making greater use of Introduced microorganisms for biological control of plant pathogens. Annual Review of Phytopathology 31: 53-80.
- Ganeshan P, Gnanamanickam SS (1987) Fungal antagonistic bacteria in rhizosphere soil Biology and biochemistry19: 35.
- Rao CVS, sachan IP, John BN (1999) Influence of fluorescent pseudomonads on growth and nodulation of lentil (Lens esculentus) in Fusarium infested soil. Indian J Microbiol 39: 23-29.
- Jagadeesh KS (2000) Selection of rhizobacteria antagonistic to Ralstoniasolanacearum causing bacterial wilt in tomato and their biocontrol mechanisms.
- Muralidharan K, Reddy CS, Krishnadevi D, Laha GS (2004) Field application of fluorescent pseudomonads products to control blast and sheath blight diseases in rice. Journal of Mycology and olant pathology 34: 411-414.
- Neelam S, Meenu S (2003) Phosphate solubilization, exopolysaccharide production and indole acetic acid secretion by rhizobacteria isolated from Trigonella foenum-graceum. Indian J Microbiology 43: 37-40.
- Suneesh K (2004) Biodiversity of fluorescent pseudomonads in soils of moist – deciduous forests of western ghats of Uttar a Kannada district.
- Manisha T, MandJohri BN (2002) In vitro antagonistic potential of fluorescent pseudomonads and control of sheath blight of maize caused by Rhizoctonia solani. Indian J Microbiology 42: 207-214.
- Sakthival N, GnanaManickam SS (1987) Evaluation of Pseudomonas fluorescens for Suppression of Sheath Rot Disease and for Enhancement of Grain Yields in Rice (Oryza sativa L.). Appl Environ Microbiol 53: 2056-2059.
- Hill GT, Mitkowskia NA, Aldrich-Wolfeb L, Emelea LR, Jurkonie DD et al. (2000) Methods for assessing the composition and diversity of soil microbial communities. Applied soil microbiology 15: 25-36.
- Pikovskaya RI (1948) Mobilization of phosphorous in soil in connection with vital activity of some microbial species. Microbiologiya 17: 302 -370.
- Wei G, Kloepper J W, Tuzum S (1991) Induction of systemic resistance of cucumber to Colletotrichum orbiculare by select strains of plant growth promoting rhizobacteria. Phytopathology 81: 1508-1512.
- Zehnder GW, Murphy JF, Sikora EJ, Klopper JW (2001) Application of rhizobacteria for induced resistance. Eur J plant pathol 107: 39-50.
- Patil R, Jagadeesh K, Krishnaraj P, Kukarni J (1998) Bacterization of groundnut with Pseudomonas fluorescens for the control of collar rot caused by Sclerotium rolfsii Sacc. Karnataka Journal of Agricultural science 11: 423-425.
- Afsharmanesh H, Ahmadzadesh M, Nikkhah JM, Behboudi K (2010) Characterization of the antagonistic activity of a new indigenous strain of pseudomonas fluorescens isolated from onion Rhizosphere. J plant pathol 92: 187-194.
- Al-Hinai AH, Al-Sadi AM, Al-Bahry SN, Mothershaw AS, Al-Said FA, et al. (2010) Isolation and characterization of pseudomonas aeruginosa with antagonistic activity against Pythium aphanidermatum. Journal of Plant pathology 92: 653-660.
|
| Â |
| Â |